States 1 States Next Slide States Three states









- Slides: 20

States 1 States Next Slide States • Three states of matter : solid, liquid and gas • Melting : change from solid to liquid • Boiling : change from liquid to gas • Solidifying : change from liquid to solid • Condensing : change from gas to liquid

States 2 States Next Slide Latent heat of fusion • Certain amount of energy is absorbed when a substance changes from solid to liquid without a change of temperature. The same amount of energy is released when the substance changes from liquid to solid without a change of temperature. This amount of energy is called the latent heat of fusion.

States 3 States Next Slide Latent heat of vaporization • Certain amount of energy is absorbed when a substance changes from liquid to gas without a change of temperature. The same amount of energy is released, when the substance changes from gas to liquid without a change of temperature. This amount of energy is called the latent heat of vaporization.

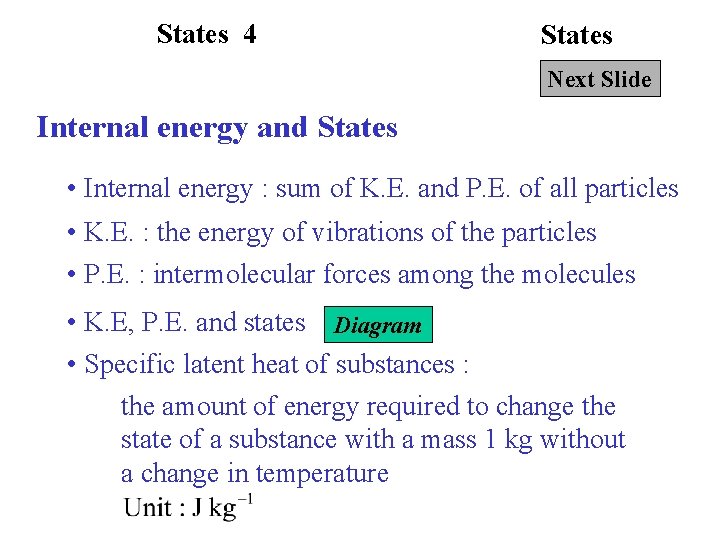
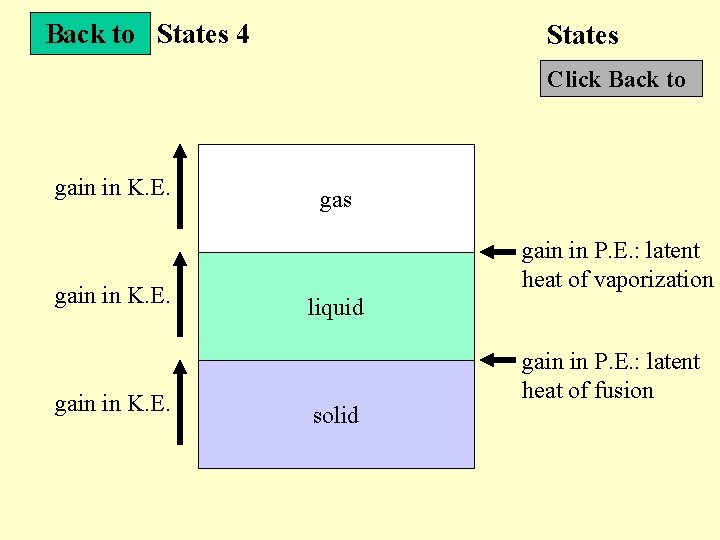
States 4 States Next Slide Internal energy and States • Internal energy : sum of K. E. and P. E. of all particles • K. E. : the energy of vibrations of the particles • P. E. : intermolecular forces among the molecules • K. E, P. E. and states Diagram • Specific latent heat of substances : the amount of energy required to change the state of a substance with a mass 1 kg without a change in temperature

States 5 States Next Slide Experiment • Experiment to find the specific latent heat of fusion of ice Diagram • Experiment to investigate the change of state Diagram • Heating curves and cooling curves Diagram • Example Diagram

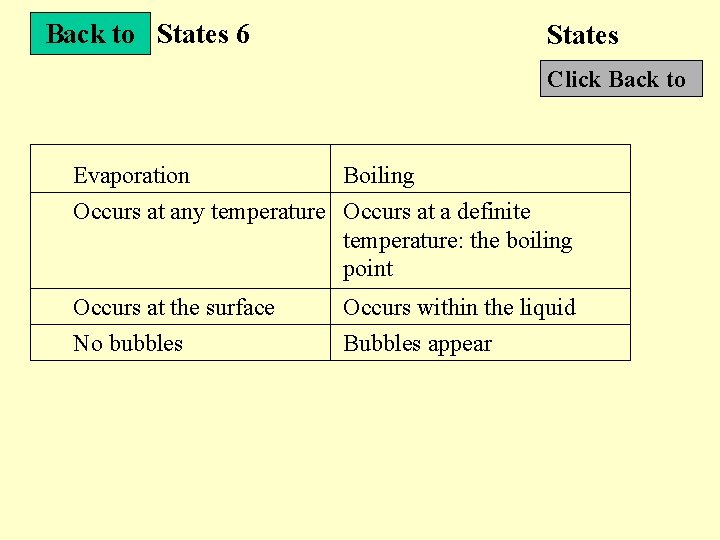
States 6 States Next Slide Evaporation and applications • Evaporation : Liquid changes to gas at a temperature below the boiling point • Differences between evaporation and boiling Diagram • Examples of latent heat Photo

END of Change of State

Back to States 4 States Click Back to gain in K. E. gas gain in P. E. : latent heat of vaporization liquid solid gain in P. E. : latent heat of fusion

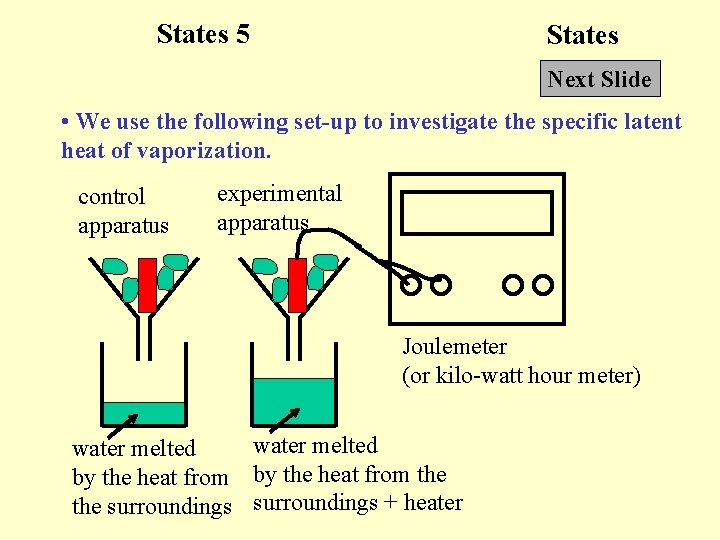
States 5 States Next Slide • We use the following set-up to investigate the specific latent heat of vaporization. control apparatus experimental apparatus Joulemeter (or kilo-watt hour meter) water melted by the heat from the surroundings + heater

Back to States 5 States Click Back to • Mass of experimental cup + water = 0. 045 kg • Mass of control cup + water = 0. 014 kg • Mass of water melted by heater only = (0. 045 0. 014) kg = 0. 031 kg • Initial Joulemeter reading = 15600 J • Final Joulemeter reading = 29800 J • Energy supplied = (29800 15600) J = 14200 J • Specific latent heat of fusion of ice = (14200 0. 031) J/kg = 458000 J/kg

States 5 States Next Slide • Some wax is placed in a test-tube with a thermometer. • The test-tube is heated until the wax melts and becomes liquid and then the test-tube is left alone. • The temperature vs time graph is plotted according to the data recorded. • We find that the central part of the curve levels off. During that duration, the liquid changes back to solid, i. e. a change of state.

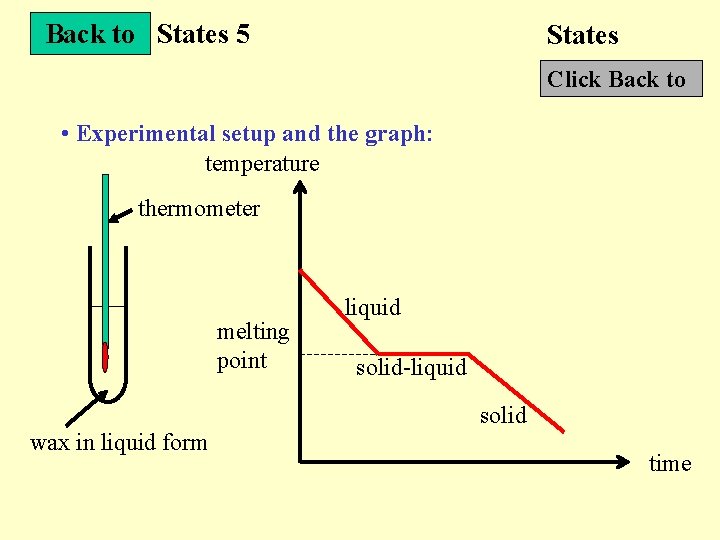
Back to States 5 States Click Back to • Experimental setup and the graph: temperature thermometer melting point liquid solid-liquid solid wax in liquid form time

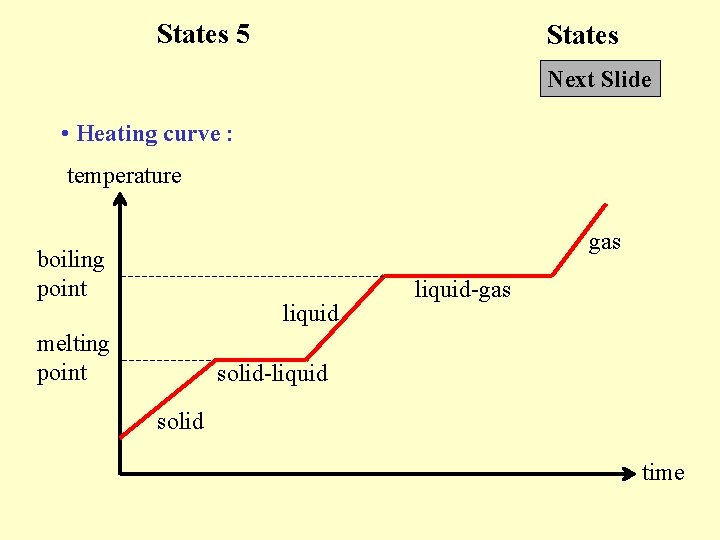
States 5 States Next Slide • Heating curve : temperature gas boiling point liquid melting point liquid-gas solid-liquid solid time

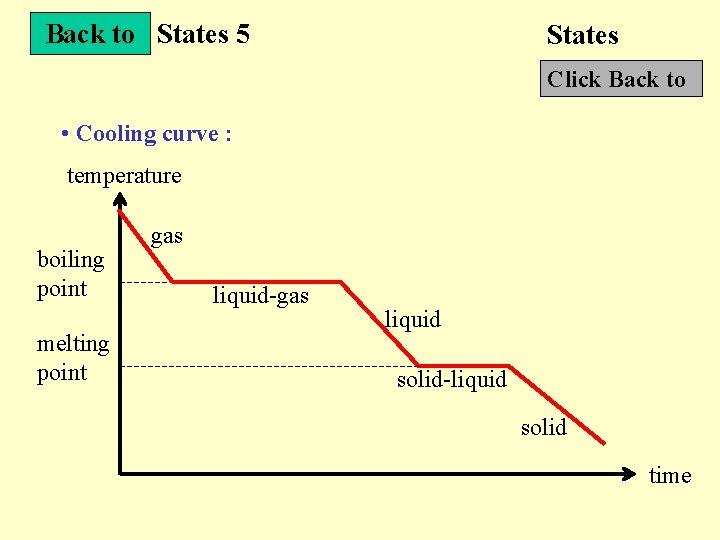
Back to States 5 States Click Back to • Cooling curve : temperature boiling point melting point gas liquid-gas liquid solid-liquid solid time

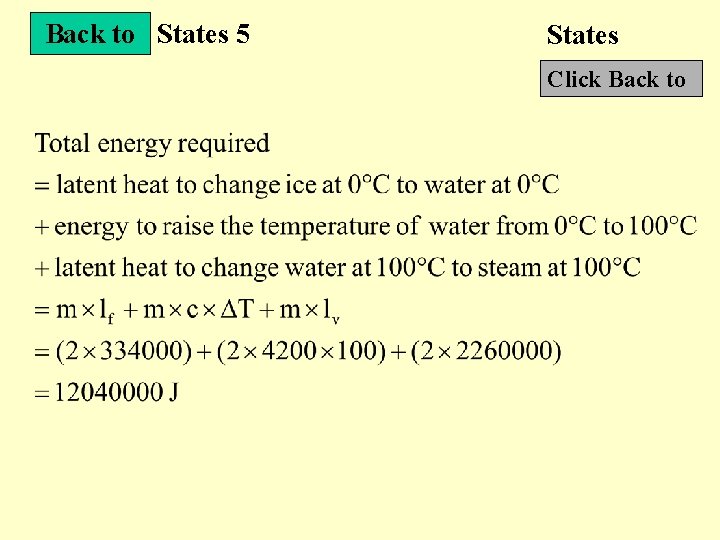
States 5 States Next Slide • What is the energy required to change a 2 kg block of ice at 0 C to steam at 100 C? (specific heat capacity of water : 4200 J/( Ckg) specific latent heat of fusion : 336000 J/kg specific latent heat of vaporization : 2280000 J/kg)

Back to States 5 States Click Back to

Back to States 6 States Click Back to Evaporation Boiling Occurs at any temperature Occurs at a definite temperature: the boiling point Occurs at the surface No bubbles Occurs within the liquid Bubbles appear

States 6 States Next Slide • Sweating : evaporation of sweat carries heat away from the body

States 6 States Next Slide • Refrigerator : Freon evaporates inside a refrigerator and carries away some heat. At the back of the refrigerator, freon vapour condenses and releases the heat.

Back to States 6 States Click Back to • You feel cold when you are out of a swimming pool in winter, though it is warm when you are in the swimming pool.