Chapter 6 Periodic Table and Periodic Law The

Chapter 6 Periodic Table and Periodic Law

The Periodic Table got its name because of the repeating pattern of chemical & physical properties. n Mendeleev ordered his periodic table with elements arranged in order of increasing atomic mass. n

Mendeleev noticed there seemed to be a repeating pattern of properties such as densities, formulas with oxygen & hydrogen, boiling or melting points every 8 or 18 elements. He called this repeating quality, periodic. (Periodic ~ according to a pattern) n He started new rows so that the elements Similar properties in eachbe aligned in having the same properties would column each column. n Holes for undiscovered elements

n He also noticed some gaps - missing elements n Based on his periodic table Mendeleev predicted the properties of the missing elements. Others tried to prove him wrong, but it turns out that he was right. Scientists soon found the missing elements and Mendeleev was very close with his predictions.
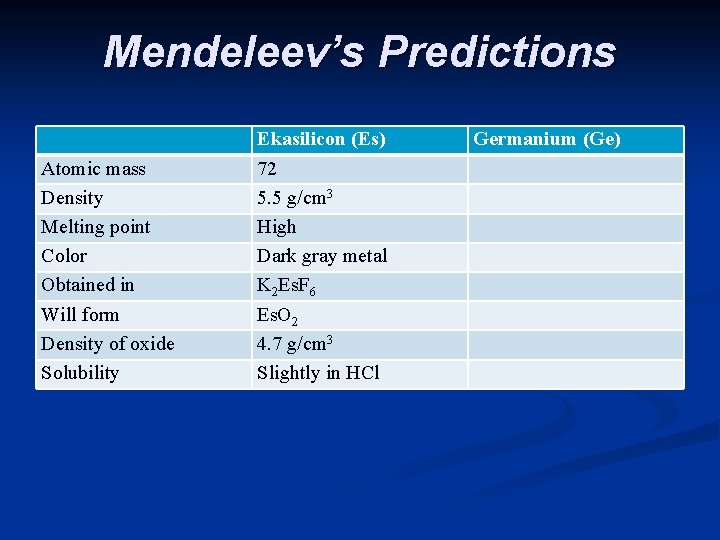
Mendeleev’s Predictions Atomic mass Density Melting point Color Obtained in Will form Density of oxide Solubility Ekasilicon (Es) 72 5. 5 g/cm 3 High Dark gray metal K 2 Es. F 6 Es. O 2 4. 7 g/cm 3 Slightly in HCl Germanium (Ge)
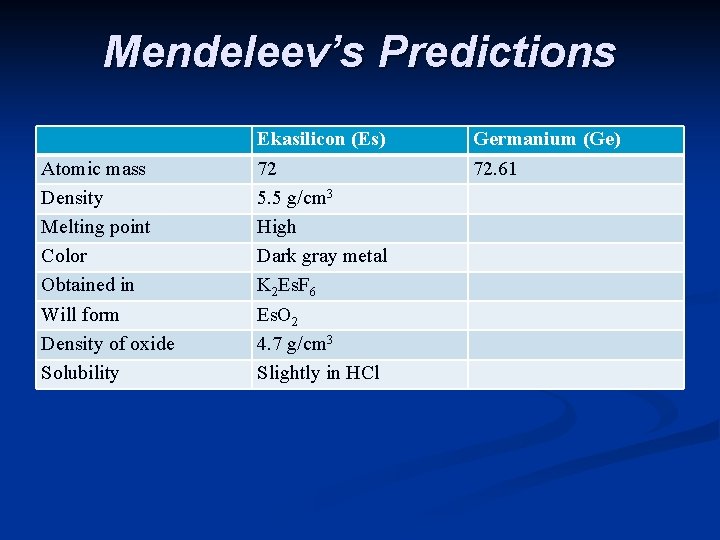
Mendeleev’s Predictions Atomic mass Density Melting point Color Obtained in Will form Density of oxide Solubility Ekasilicon (Es) 72 5. 5 g/cm 3 High Dark gray metal K 2 Es. F 6 Es. O 2 4. 7 g/cm 3 Slightly in HCl Germanium (Ge) 72. 61
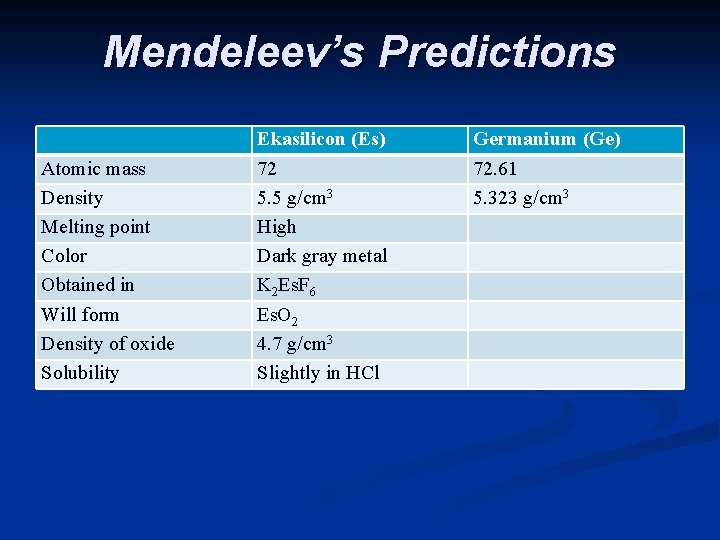
Mendeleev’s Predictions Atomic mass Density Melting point Color Obtained in Will form Density of oxide Solubility Ekasilicon (Es) 72 5. 5 g/cm 3 High Dark gray metal K 2 Es. F 6 Es. O 2 4. 7 g/cm 3 Slightly in HCl Germanium (Ge) 72. 61 5. 323 g/cm 3
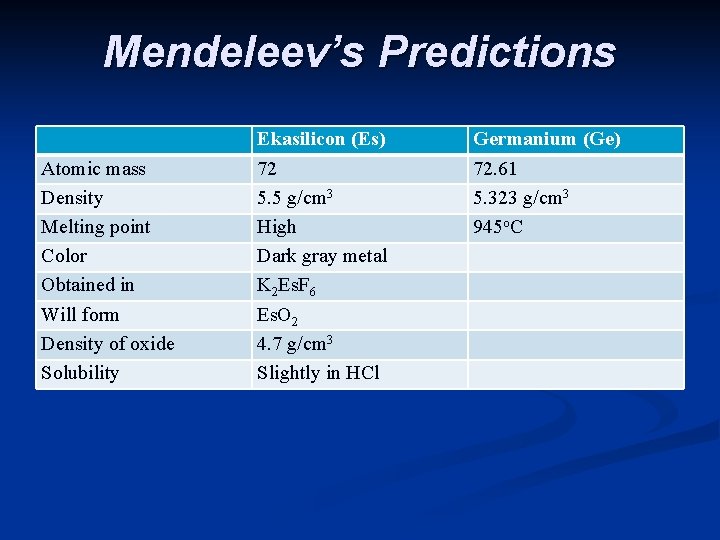
Mendeleev’s Predictions Atomic mass Density Melting point Color Obtained in Will form Density of oxide Solubility Ekasilicon (Es) 72 5. 5 g/cm 3 High Dark gray metal K 2 Es. F 6 Es. O 2 4. 7 g/cm 3 Slightly in HCl Germanium (Ge) 72. 61 5. 323 g/cm 3 945 o. C
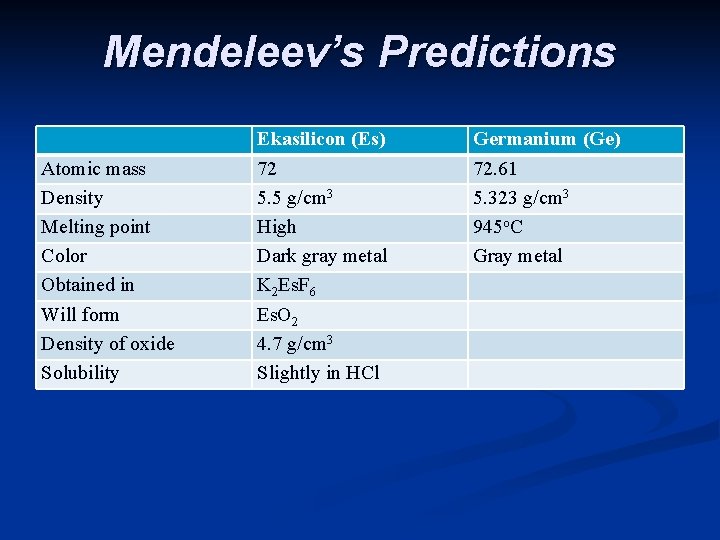
Mendeleev’s Predictions Atomic mass Density Melting point Color Obtained in Will form Density of oxide Solubility Ekasilicon (Es) 72 5. 5 g/cm 3 High Dark gray metal K 2 Es. F 6 Es. O 2 4. 7 g/cm 3 Slightly in HCl Germanium (Ge) 72. 61 5. 323 g/cm 3 945 o. C Gray metal
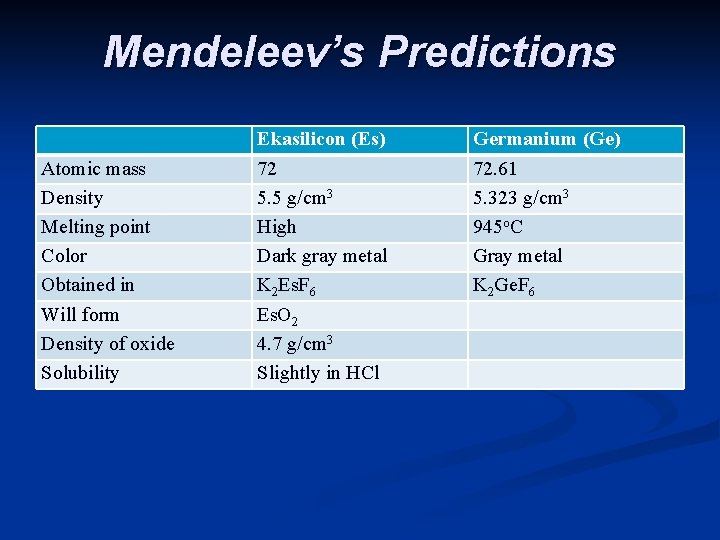
Mendeleev’s Predictions Atomic mass Density Melting point Color Obtained in Will form Density of oxide Solubility Ekasilicon (Es) 72 5. 5 g/cm 3 High Dark gray metal K 2 Es. F 6 Es. O 2 4. 7 g/cm 3 Slightly in HCl Germanium (Ge) 72. 61 5. 323 g/cm 3 945 o. C Gray metal K 2 Ge. F 6
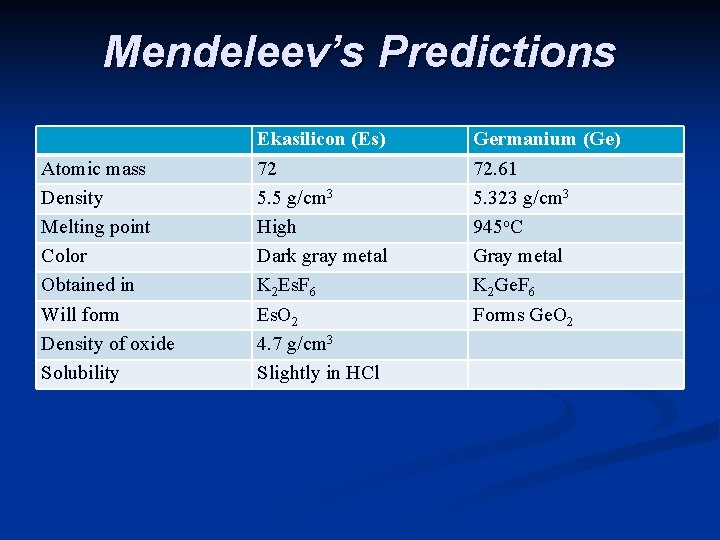
Mendeleev’s Predictions Atomic mass Density Melting point Color Obtained in Will form Density of oxide Solubility Ekasilicon (Es) 72 5. 5 g/cm 3 High Dark gray metal K 2 Es. F 6 Es. O 2 4. 7 g/cm 3 Slightly in HCl Germanium (Ge) 72. 61 5. 323 g/cm 3 945 o. C Gray metal K 2 Ge. F 6 Forms Ge. O 2
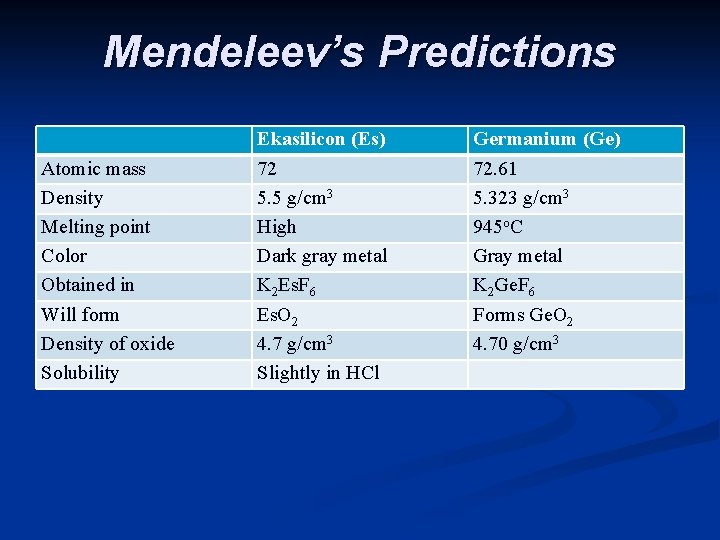
Mendeleev’s Predictions Atomic mass Density Melting point Color Obtained in Will form Density of oxide Solubility Ekasilicon (Es) 72 5. 5 g/cm 3 High Dark gray metal K 2 Es. F 6 Es. O 2 4. 7 g/cm 3 Slightly in HCl Germanium (Ge) 72. 61 5. 323 g/cm 3 945 o. C Gray metal K 2 Ge. F 6 Forms Ge. O 2 4. 70 g/cm 3
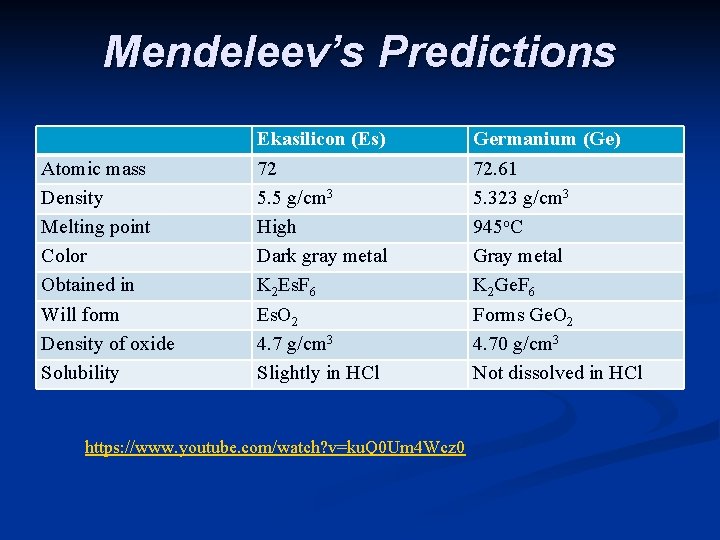
Mendeleev’s Predictions Atomic mass Density Melting point Color Obtained in Will form Density of oxide Solubility Ekasilicon (Es) 72 5. 5 g/cm 3 High Dark gray metal K 2 Es. F 6 Es. O 2 4. 7 g/cm 3 Slightly in HCl https: //www. youtube. com/watch? v=ku. Q 0 Um 4 Wcz 0 Germanium (Ge) 72. 61 5. 323 g/cm 3 945 o. C Gray metal K 2 Ge. F 6 Forms Ge. O 2 4. 70 g/cm 3 Not dissolved in HCl
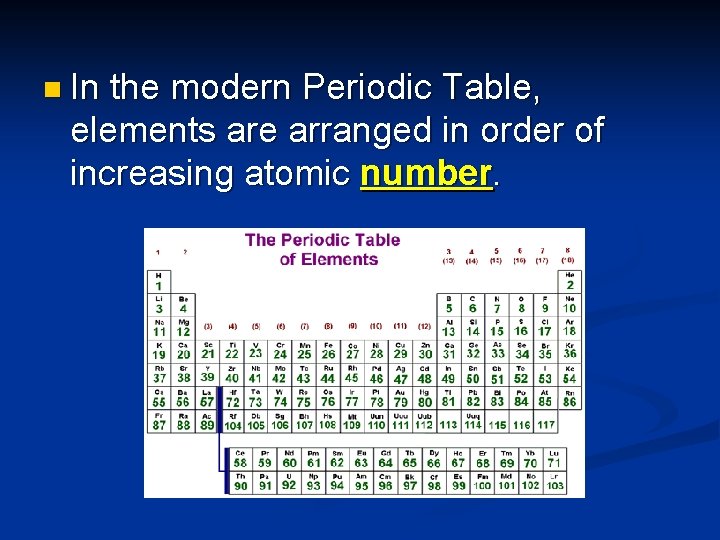
n In the modern Periodic Table, elements are arranged in order of increasing atomic number.
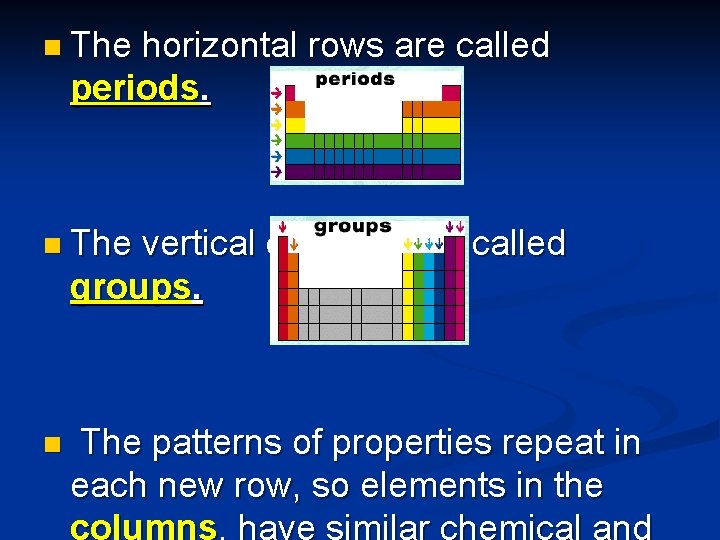
n The horizontal rows are called periods. n The vertical columns are called groups. n The patterns of properties repeat in each new row, so elements in the
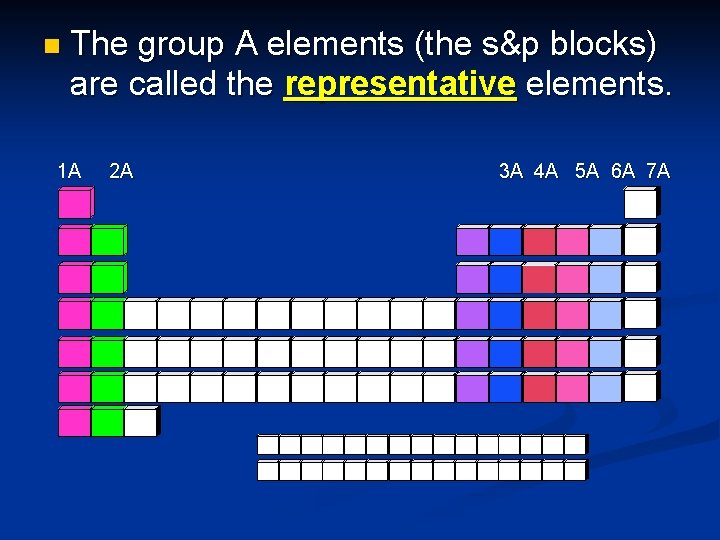
n The group A elements (the s&p blocks) are called the representative elements. 1 A 2 A 8 A 3 A 4 A 5 A 6 A 7 A
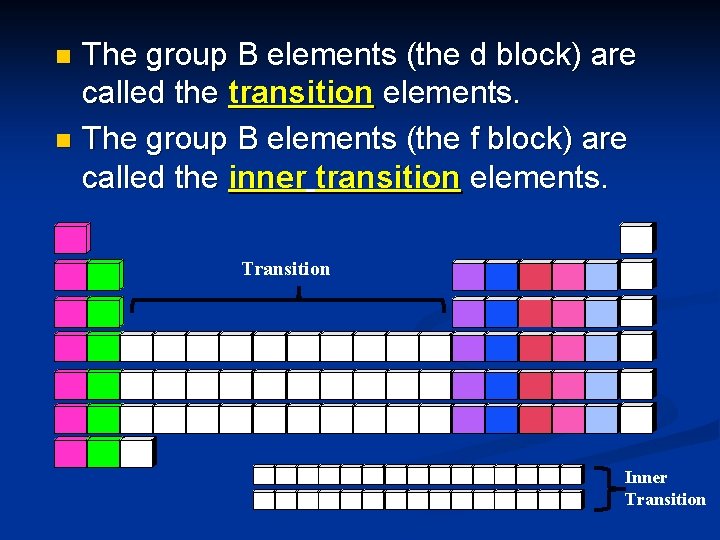
The group B elements (the d block) are called the transition elements. n The group B elements (the f block) are called the inner transition elements. n Transition Inner Transition
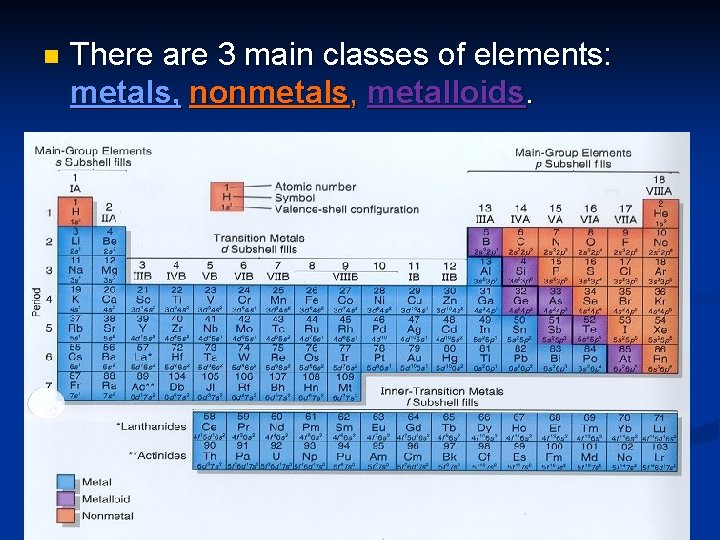
n There are 3 main classes of elements: metals, nonmetals, metalloids.
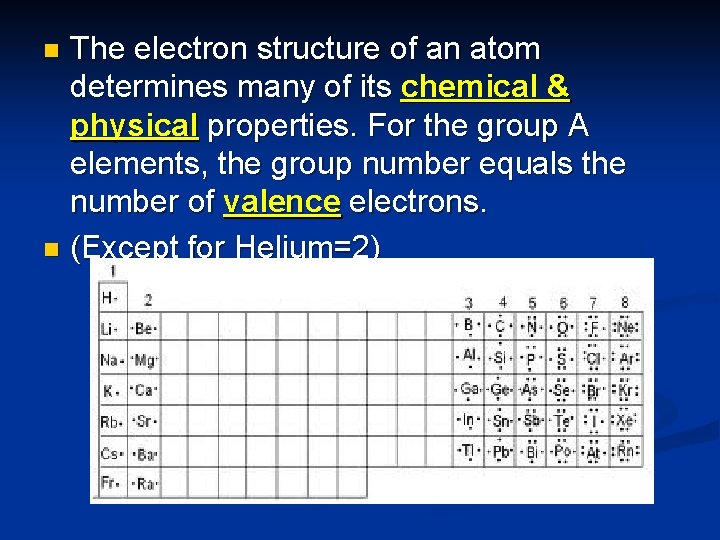
The electron structure of an atom determines many of its chemical & physical properties. For the group A elements, the group number equals the number of valence electrons. n (Except for Helium=2) n
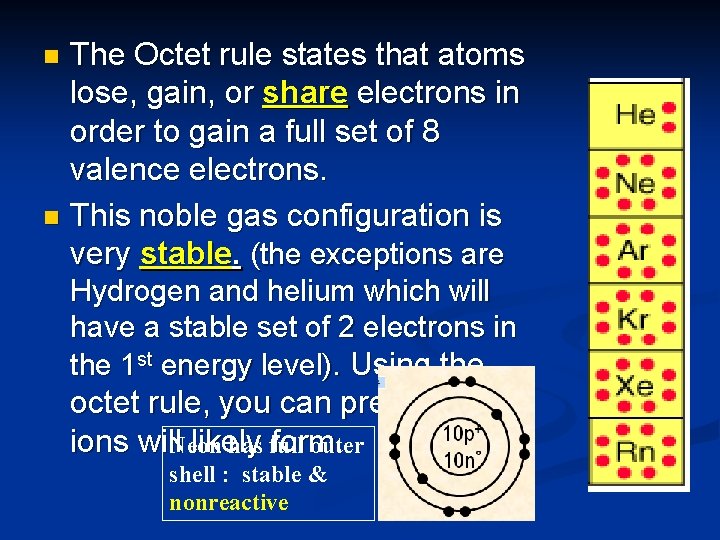
The Octet rule states that atoms lose, gain, or share electrons in order to gain a full set of 8 valence electrons. n This noble gas configuration is very stable. (the exceptions are n Hydrogen and helium which will have a stable set of 2 electrons in the 1 st energy level). Using the octet rule, you can predict which ions will. Neon likely form. has full outer shell : stable & nonreactive
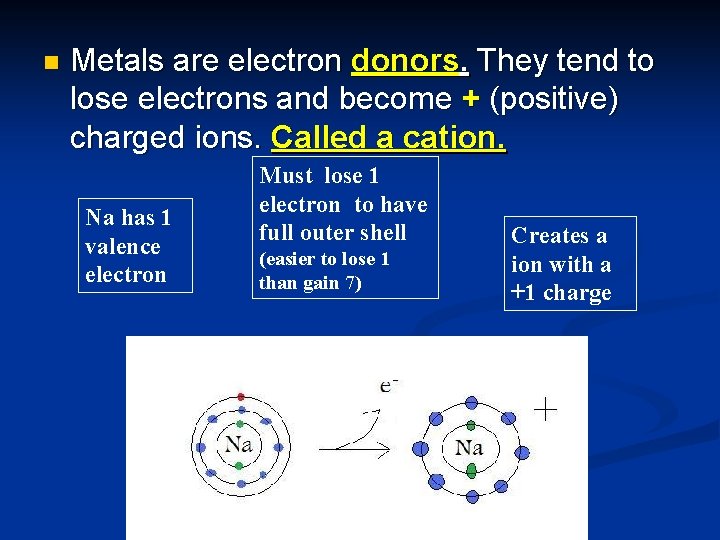
n Metals are electron donors. They tend to lose electrons and become + (positive) charged ions. Called a cation. Na has 1 valence electron Must lose 1 electron to have full outer shell (easier to lose 1 than gain 7) Creates a ion with a +1 charge
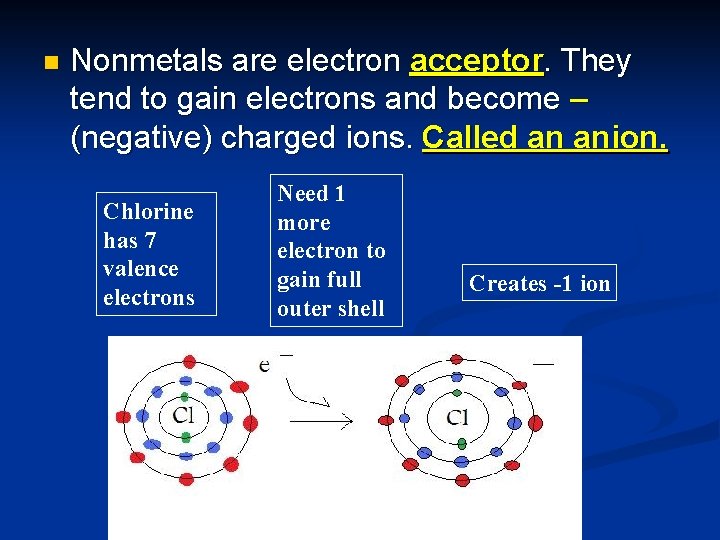
n Nonmetals are electron acceptor. They tend to gain electrons and become – (negative) charged ions. Called an anion. Chlorine has 7 valence electrons Need 1 more electron to gain full outer shell Creates -1 ion
Periodic trends n Atomic radius: Tends to decrease across the period. Electrons are being added in the same energy level so increased attraction between the larger number of + protons pulls the – electrons closer.
Periodic trends n Atomic radius: Tends to increase down the group, because you are adding energy levels, which shield the valence electrons from the pull of the nucleus.

Ionic radius n Losing electrons produce + charged ions, which are smaller than the parent atom.
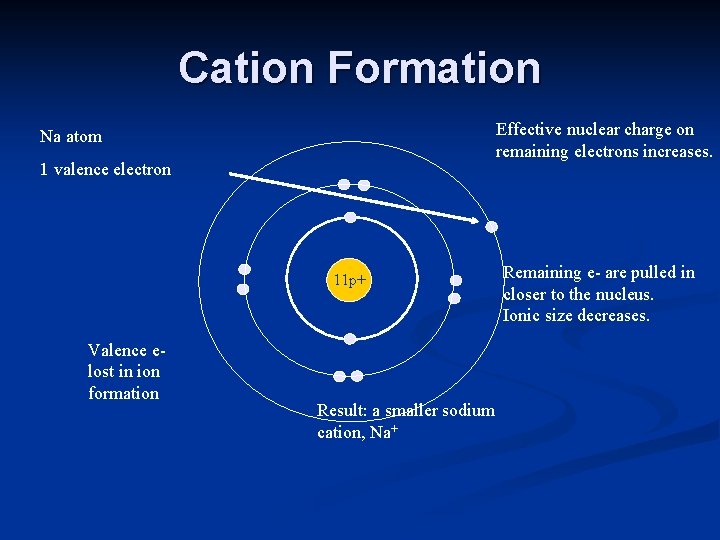
Cation Formation Effective nuclear charge on remaining electrons increases. Na atom 1 valence electron 11 p+ Valence elost in ion formation Result: a smaller sodium cation, Na+ Remaining e- are pulled in closer to the nucleus. Ionic size decreases.

Ionic radius n When atoms gain electrons, they become – charged ions, which are larger than the parent atom.
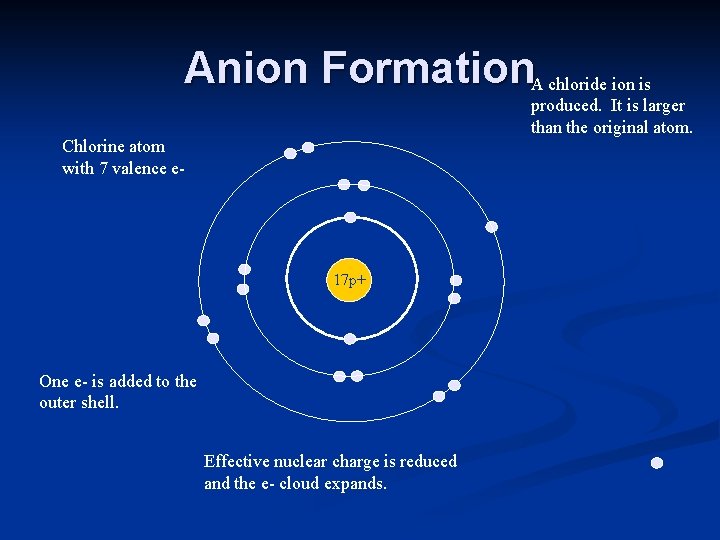
Anion Formation. A chloride ion is produced. It is larger than the original atom. Chlorine atom with 7 valence e- 17 p+ One e- is added to the outer shell. Effective nuclear charge is reduced and the e- cloud expands.
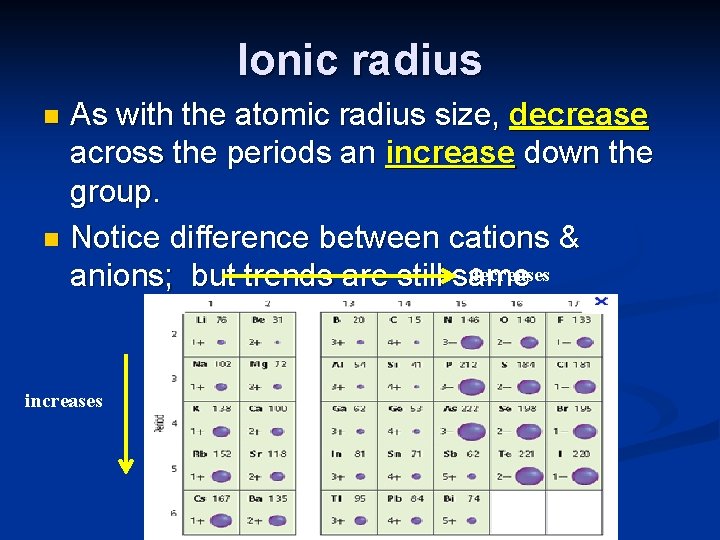
Ionic radius As with the atomic radius size, decrease across the periods an increase down the group. n Notice difference between cations & decreases anions; but trends are still same n increases

Ionic Radius – size as compared to the neutral element Positive cations are always smaller than neutral atom (removal of e ) often remaining e are in a lower energy level. n Na 1 s 22 p 63 s 1 Na 1+ 1 s 22 p 6 Anions get bigger than neutral atom. Adding e creates more e repulsion. n Cl 1 s 22 p 63 s 23 p 5 Cl 1 1 s 22 p 63 s 23 p 6

Ionization energy n Is the amount of energy required to pull the 1 st valence electron away from the atom. n Elements with high ionization energy are [unlikely, likely] to lose electrons. Atoms with low ionization energy [easily, don’t really] lose electrons
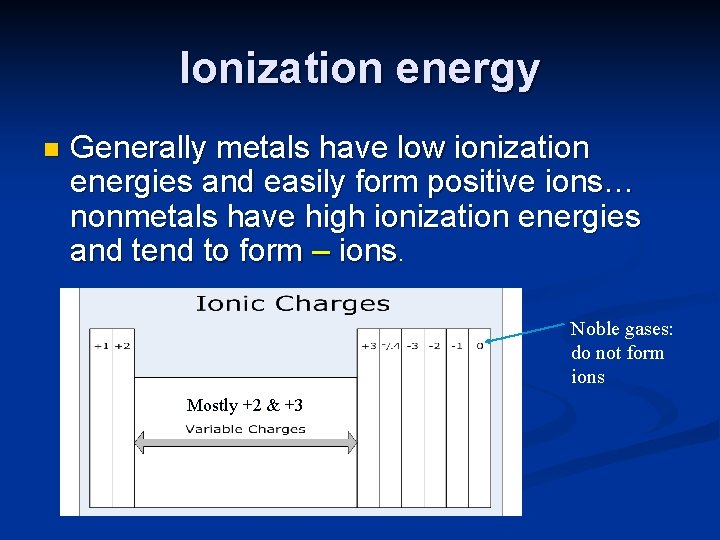
Ionization energy n Generally metals have low ionization energies and easily form positive ions… nonmetals have high ionization energies and tend to form – ions. Noble gases: do not form ions Mostly +2 & +3
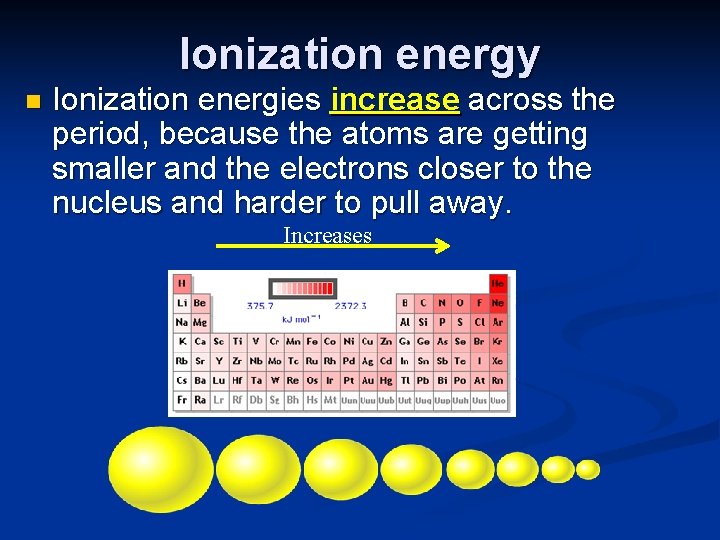
Ionization energy n Ionization energies increase across the period, because the atoms are getting smaller and the electrons closer to the nucleus and harder to pull away. Increases
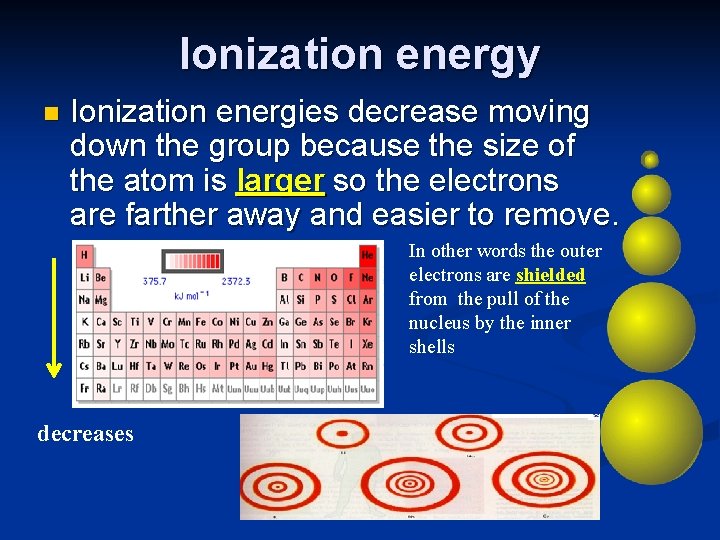
Ionization energy n Ionization energies decrease moving down the group because the size of the atom is larger so the electrons are farther away and easier to remove. In other words the outer electrons are shielded from the pull of the nucleus by the inner shells decreases

Electron Affinity E. A. the amount of energy released when an atom gains an e-. The opposite process of ionization. (removing of e-) “affinity” means fondness. ~ E. A. increases across a period. Why? Nonmetals give off more energy when they gain an e- than metals do. ~ E. A. decreases down a family. Why? b/c of the larger # of e- less energy is given off w/the
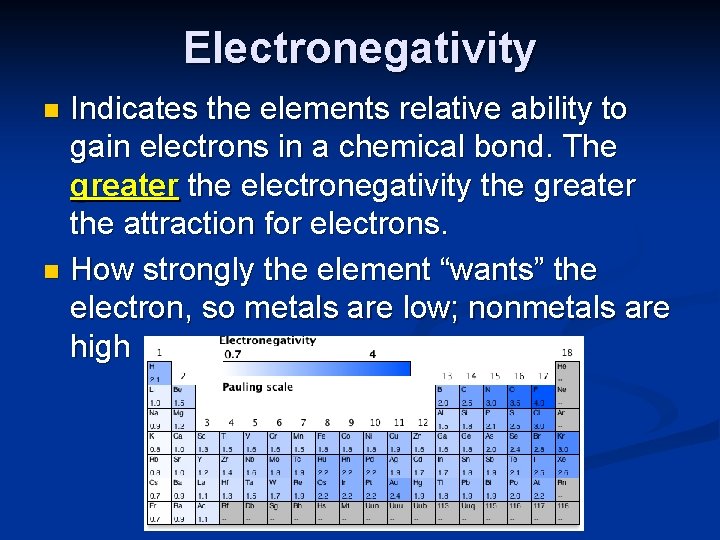
Electronegativity Indicates the elements relative ability to gain electrons in a chemical bond. The greater the electronegativity the greater the attraction for electrons. n How strongly the element “wants” the electron, so metals are low; nonmetals are high n
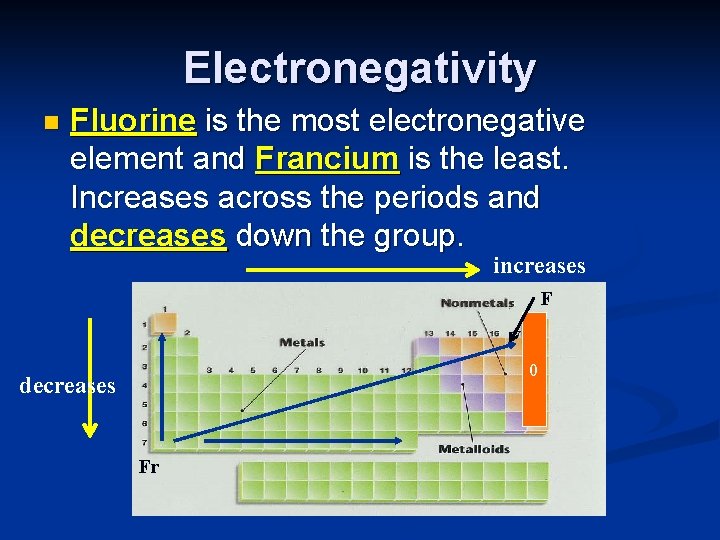
Electronegativity n Fluorine is the most electronegative element and Francium is the least. Increases across the periods and decreases down the group. increases F 0 decreases Fr

n Two most important trends are atomic radius and electronegativity. n *For all trends except size & #, the closer you are to F, the greater the trend
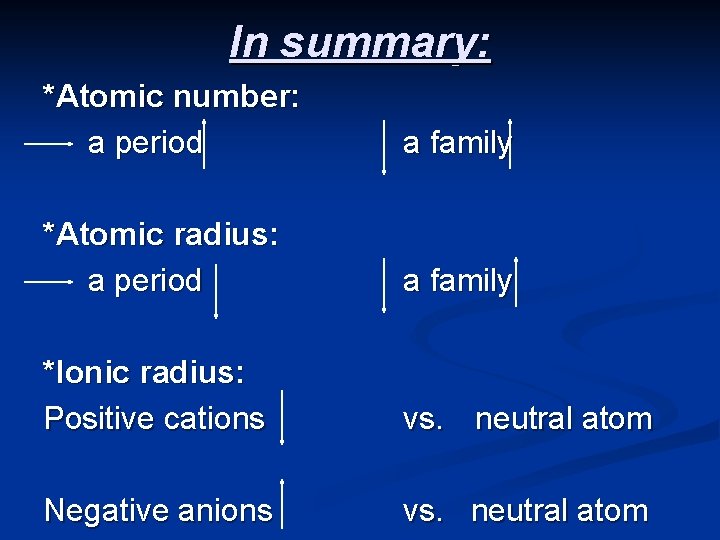
In summary: *Atomic number: a period a family *Atomic radius: a period a family *Ionic radius: Positive cations vs. neutral atom Negative anions vs. neutral atom
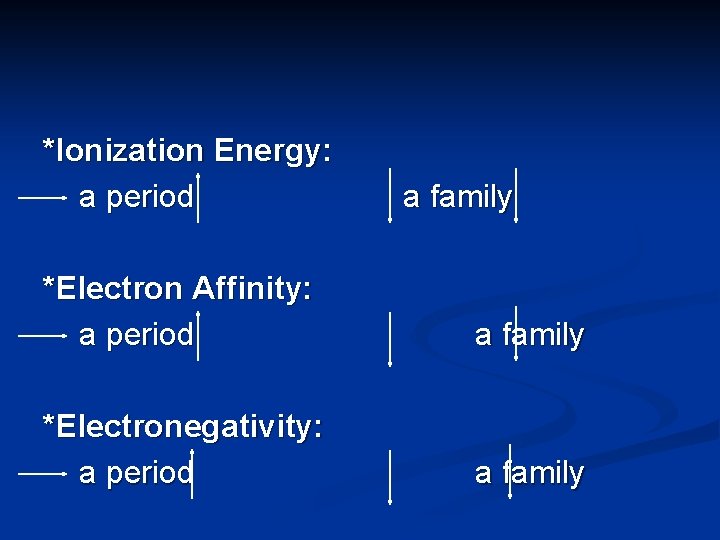
*Ionization Energy: a period a family *Electron Affinity: a period a family *Electronegativity: a period a family

Some specific groups: Group IA Alkali metals: Li, Na, K, Rb, Cs, Fr n In pure state have a silvery appearance and are soft enough to cut with a knife. Yet, alkali metals are so reactive they are not found in nature as free elements. Combine vigorously w/most nonmetals. Usually stored in Kerosene.

Group IIA Alkaline earth metals: Be, Mg, Ca, Sr, Ba, Ra n Have 2 e- in outermost level. They are harder, denser, and stronger than alkali metals. Although they are less reactive than group IA, they are too reactive to be found alone in nature.

Group VII A Halogens: F, Cl, Br, I, At n Most reactive nonmetals. React vigorously w/most metals to form a type of compound known as salts. Has 7 e- in its outer energy level.

Group VIII A Noble Gases: He, Ne, Ar, Kr, Xe, Rn n These are the least reactive of all the elements. Have very stable electron configurations. For many years, the noble gases were believed to be chemically unreactive, yet in the lab inert gas compounds can be synthesized.
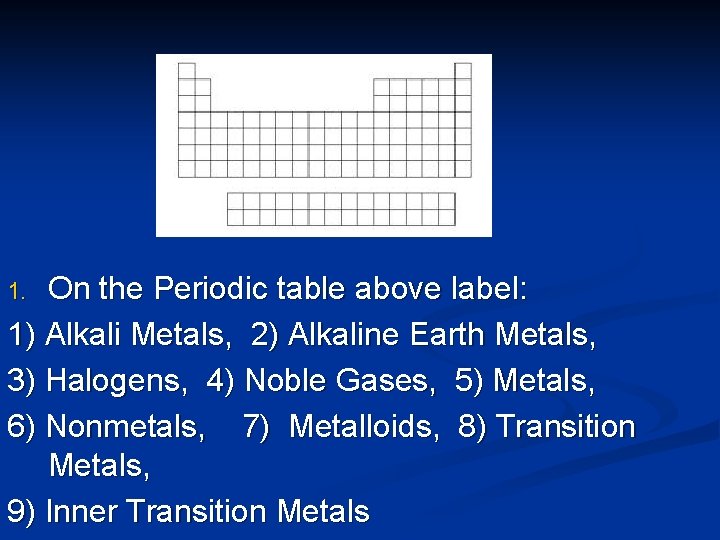
On the Periodic table above label: 1) Alkali Metals, 2) Alkaline Earth Metals, 3) Halogens, 4) Noble Gases, 5) Metals, 6) Nonmetals, 7) Metalloids, 8) Transition Metals, 9) Inner Transition Metals 1.
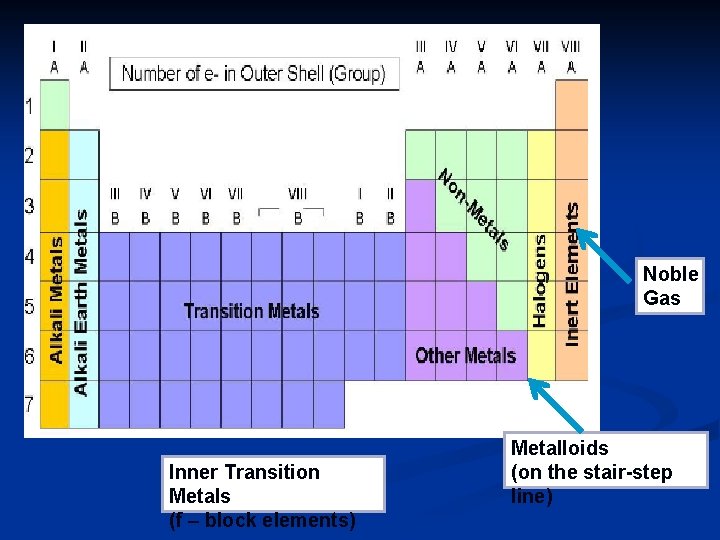
Noble Gas Inner Transition Metals (f – block elements) Metalloids (on the stair step line)

2. Explain why the word periodic is applied to the table of elements. The pattern of properties repeats periodically with each new row

3. Why do elements in a group (vertical column) in the periodic table exhibit similar chemical properties? They have the same arrangement of valence electrons

4. What chemical property is common to the elements in group 8 A. Explain why. Group 8 A = noble gases = chemically unreactive because they have a full set of 8 (octet) valence electrons

5. In terms of electron configuration, what does the group number of the A-groups tell you? The A-group number = the number of valence electrons

6. Describe the relationship between the electronegativity value of an element and the tendency of that element to gain or lose electrons when forming a chemical bond. The higher the electronegativiy, the greater the tendency to gain an electron.
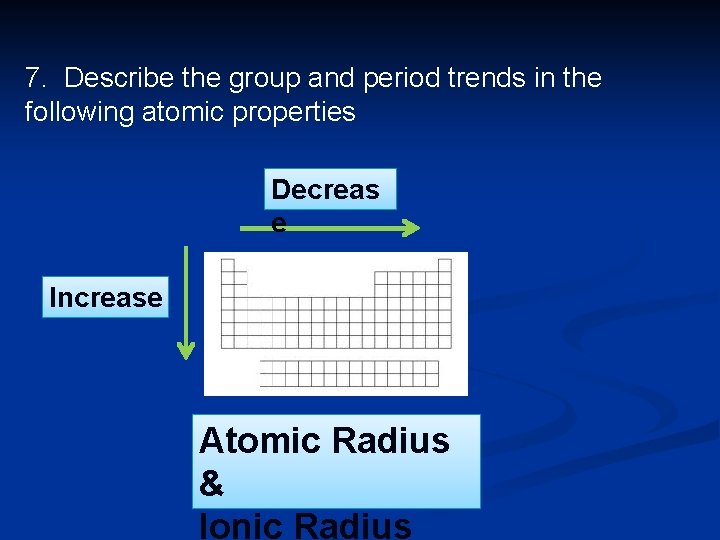
7. Describe the group and period trends in the following atomic properties Decreas e Increase Atomic Radius & Ionic Radius
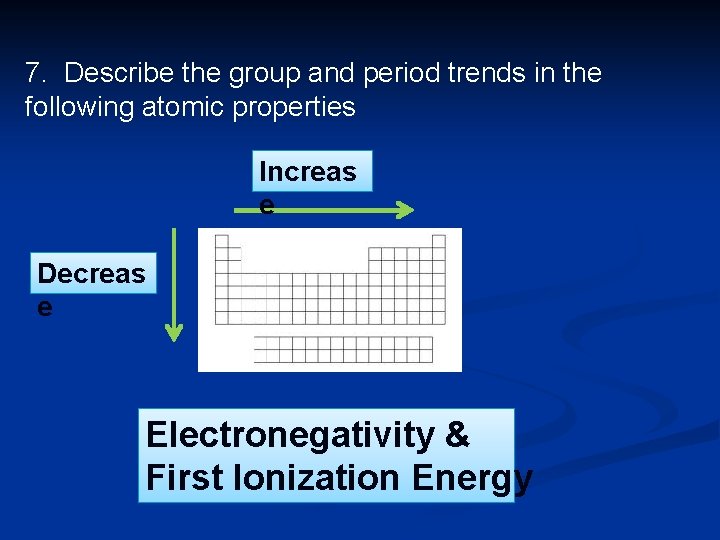
7. Describe the group and period trends in the following atomic properties Increas e Decreas e Electronegativity & First Ionization Energy
- Slides: 53