Chapter 14 Periodic Trends Modern Chemistry Holt Rinehart







- Slides: 14

Chapter 14: Periodic Trends Modern Chemistry; Holt, Rinehart, & Winston

Dmitri Mendeleev (1834 -1907) l l l Russian chemist Arranged his periodic table according to atomic mass so that elements with similar properties were in the same group Predicted the properties of elements that had not yet been discovered using his periodic table

Henry Moseley (1887 -1915) l l English chemist Proved Mendeleev’s arrangement of the periodic table to be correct – only, the periodic table was arranged according to atomic number, not atomic mass

The Periodic Law l States that when elements are arranged in order of increasing atomic number, their physical and chemical properties show a periodic pattern

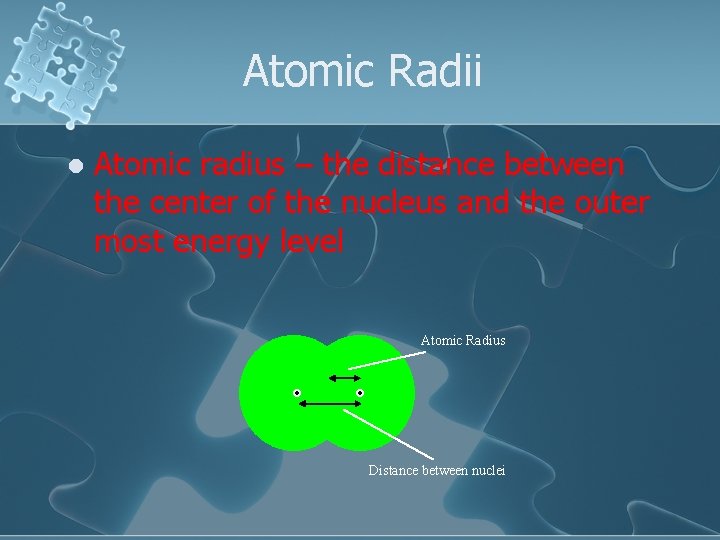
Atomic Radii l Atomic radius – the distance between the center of the nucleus and the outer most energy level Atomic Radius Distance between nuclei

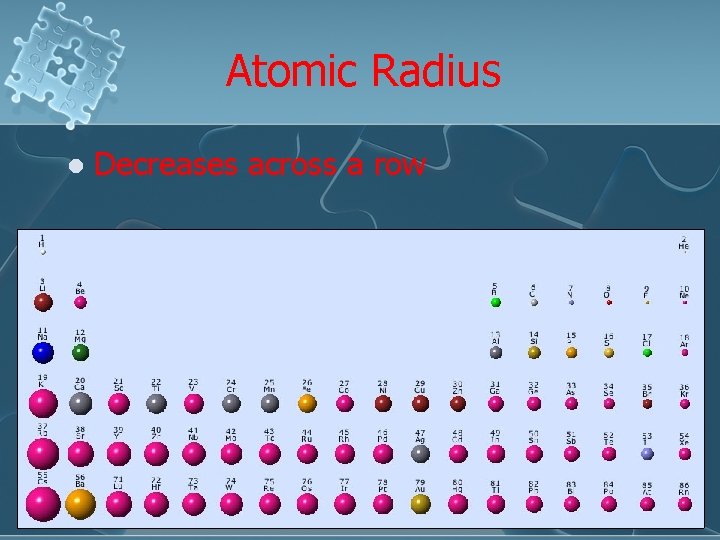
Atomic Radius l Decreases across a row

Why? Protons are added to the nucleus moving across a period from left to right l This increases the charge of the nucleus (effective nuclear charge – Zeff) l As a result, it compacts the atom l

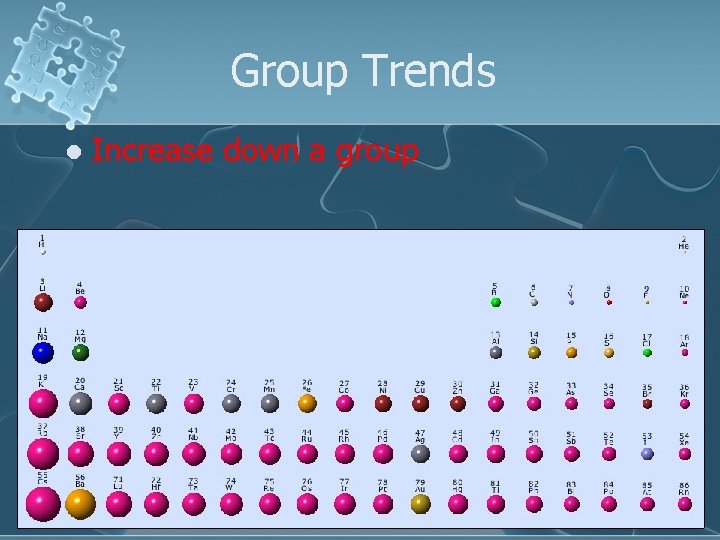
Group Trends l Increase down a group

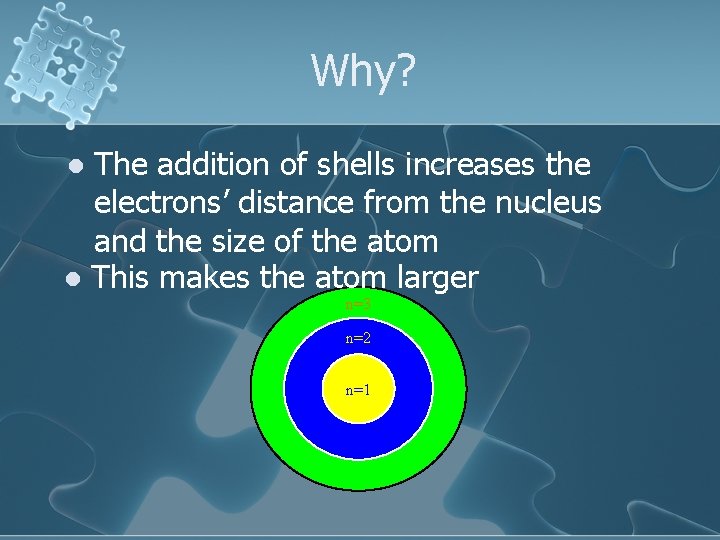
Why? The addition of shells increases the electrons’ distance from the nucleus and the size of the atom l This makes the atom larger l n=3 n=2 n=1

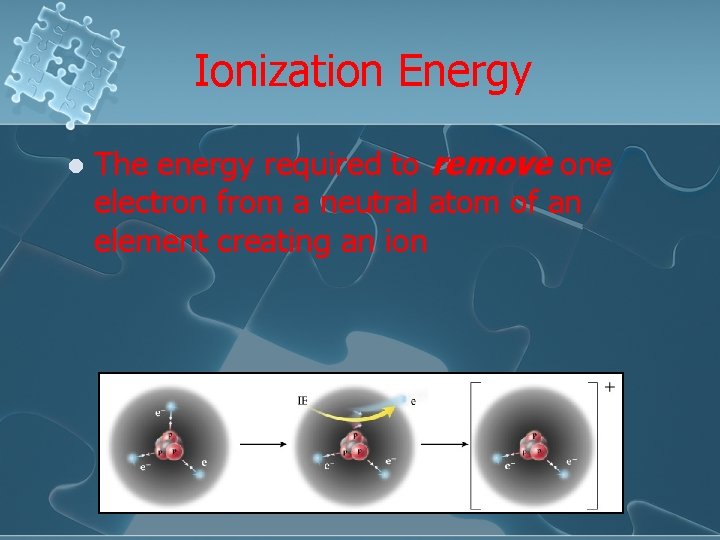
Ionization Energy l The energy required to remove one electron from a neutral atom of an element creating an ion

Period Trends Increase across a row l Why? l Zeff increases across the period l There is stronger attraction between the positive and negative particles more difficult to remove them. l

Group Trends Decrease down the column l Why? l l Electrons are far away from positive nucleus, making it easier to remove

Electronegativity l A measure of the ability of an atom in a chemical compound to attract a bonding pair of electrons l A MEASURE OF GREEDINESS!

Trends l Increase across a period l Decrease down a group l EXCEPTIONS: NOBLE GAS (GROUP 18). They do not have an electronegativity value because they do not form chemical compounds.
 Trends in periodic table
Trends in periodic table How to find out group and period of an element
How to find out group and period of an element William henry rinehart
William henry rinehart Amanda rinehart
Amanda rinehart Chapter 6 the periodic table and periodic law
Chapter 6 the periodic table and periodic law The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Direction of dipole moment
Direction of dipole moment Buoyancy force in fluid mechanics
Buoyancy force in fluid mechanics Holt physics chapter 2
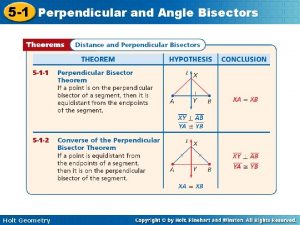
Holt physics chapter 2 Holt geometry chapter 5
Holt geometry chapter 5 Ap chemistry chapter 7 periodic properties of the elements
Ap chemistry chapter 7 periodic properties of the elements The atomic radius in periodic table
The atomic radius in periodic table Trends in the periodic table labster
Trends in the periodic table labster Ultimate periodic table cheat sheet
Ultimate periodic table cheat sheet