The Periodic Table of Elements Periodic Table Something
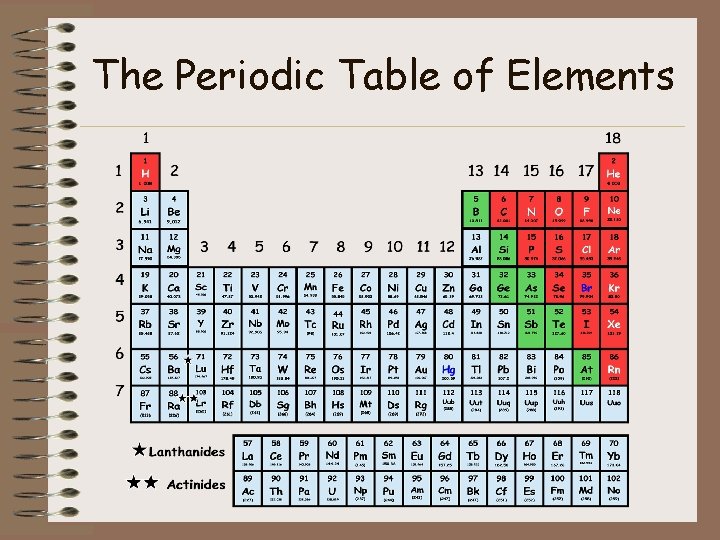
The Periodic Table of Elements
Periodic Table • Something periodic occurs at regular or at least generally predictable intervals • Periodic Table of Elements – a table of the elements, arranged by atomic number, that shows the patterns in their properties

Element • A pure substance made up of one kind of atom that cannot be broken down into simpler substances by physical or chemical means • 94 occur naturally on earth • 24 were synthesized (made) by scientists http: //www. youtube. com/watch? v=z. UDDi. Wt. Ft. EM

Dmitri Mendeleev • In the 1860’s he devised a periodic table where the elements were ordered by their atomic masses • He did this by grouping elements together according to their similarities Image taken from: http: //jscms. jrn. columbia. edu/cns/2006 -04 -18/fido-luxuriantflowinghair/mendeleev/

Mendeleev’s Published Periodic Table of Elements Why do you think there are question marks here? Image taken from: http: //www. chemsoc. org/networks/learnnet/periodictable/post 16/develop/mendeleev. htm
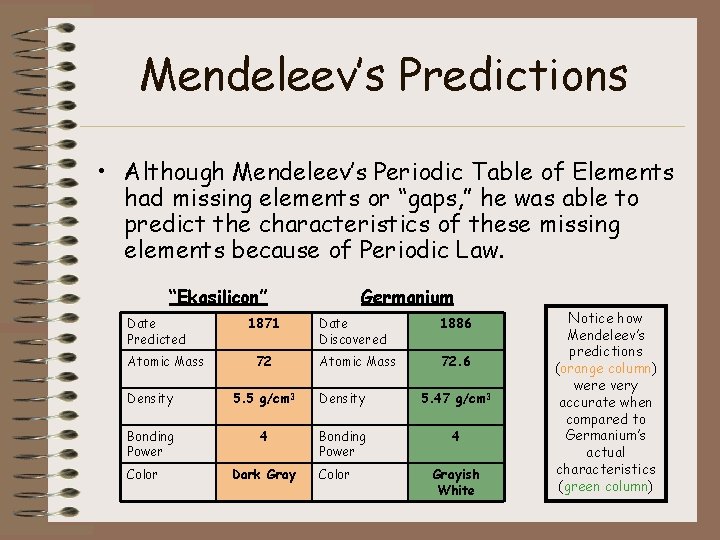
Mendeleev’s Predictions • Although Mendeleev’s Periodic Table of Elements had missing elements or “gaps, ” he was able to predict the characteristics of these missing elements because of Periodic Law. “Ekasilicon” Date Predicted Atomic Mass 1871 72 Germanium Date Discovered 1886 Atomic Mass 72. 6 Density 5. 5 g/cm 3 Density 5. 47 g/cm 3 Bonding Power 4 Color Dark Gray Color Grayish White Notice how Mendeleev’s predictions (orange column) were very accurate when compared to Germanium’s actual characteristics (green column)

Henry Moseley • In 1914, his work led to a revision of the periodic table by rearranging the elements by their atomic numbers (not atomic masses) • He concluded that the number of protons in an atom is its atomic number Image taken from: http: //dewey. library. upenn. edu/sceti/smith/
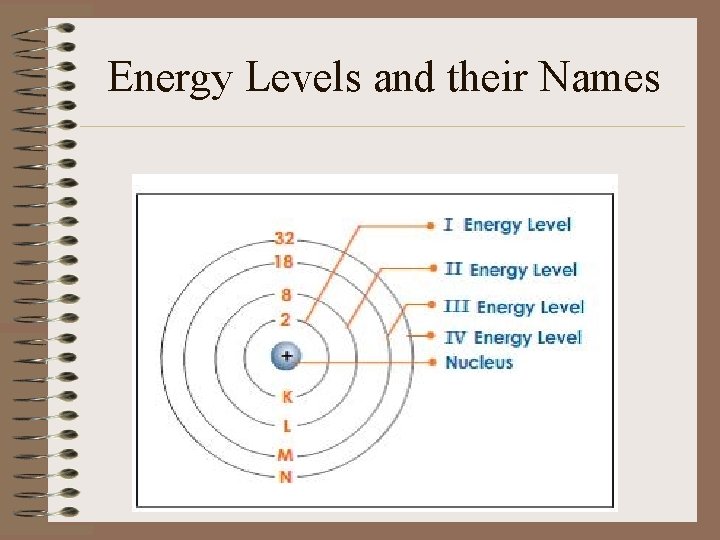
Energy Levels and their Names
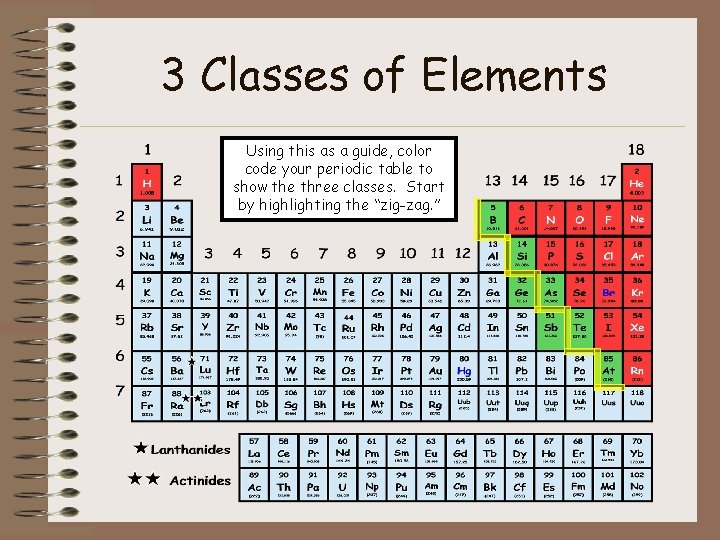
3 Classes of Elements Classas a guide, Color color Using this Metal code your periodic table to Non-Metal show the three classes. Start Metalloid by highlighting the “zig-zag. ”

Metals Location • Found on the left of the zigzag line/staircase on the periodic table (exception Hydrogen) Chemical Properties • Have few electrons in their outer energy level, thus lose electrons easily Physical Properties • Ductile (able to be drawn out into a thin wire), good conductors, malleable (able to be hammered without breaking or cracking), shiny, most are solid @ room temperature 79 Au 196. 967 11 Na 22. 990 Image taken from: http: //chemistry. about. com/od/periodictableelements/ig/El ement-Photo-Gallery. --98/Sodium. htm What metal is not a solid @ room temperature?
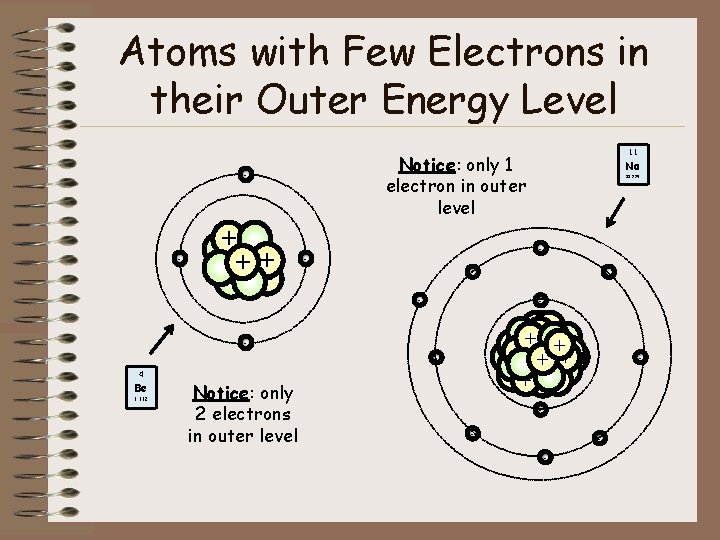
Atoms with Few Electrons in their Outer Energy Level Notice: only 1 electron in outer level - + + ++ + - Notice: only 2 electrons in outer level - - Be 22. 990 - 9. 012 Na - - + 4 11 - - -
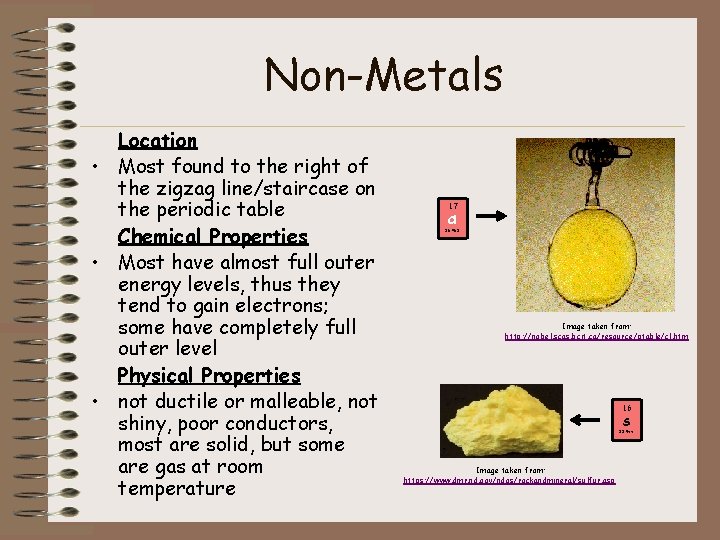
Non-Metals Location • Most found to the right of the zigzag line/staircase on the periodic table Chemical Properties • Most have almost full outer energy levels, thus they tend to gain electrons; some have completely full outer level Physical Properties • not ductile or malleable, not shiny, poor conductors, most are solid, but some are gas at room temperature 17 Cl 35. 453 Image taken from: http: //nobel. scas. bcit. ca/resource/ptable/cl. htm 16 S 32. 066 Image taken from: https: //www. dmr. nd. gov/ndgs/rockandmineral/sulfur. asp
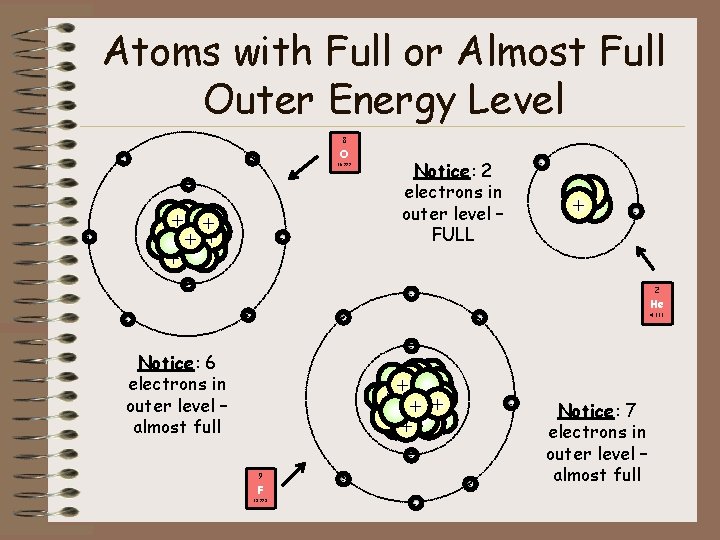
Atoms with Full or Almost Full Outer Energy Level 8 - O - 15. 999 - ++ + + + - - - Notice: 2 electrons in outer level – FULL - + + - 2 - He - 4. 003 - Notice: 6 electrons in outer level – almost full +++ + - 9 F 18. 998 - - Notice: 7 electrons in outer level – almost full
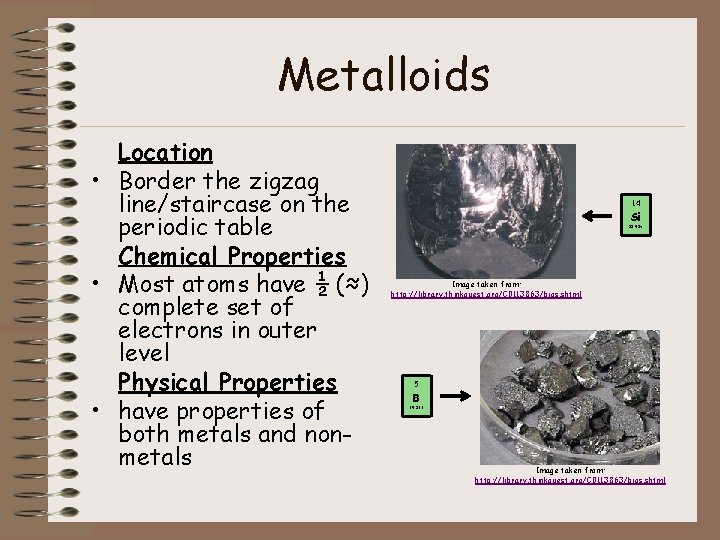
Metalloids Location • Border the zigzag line/staircase on the periodic table Chemical Properties • Most atoms have ½ (≈) complete set of electrons in outer level Physical Properties • have properties of both metals and nonmetals 14 Si 28. 086 Image taken from: http: //library. thinkquest. org/C 0113863/bios. shtml 5 B 10. 811 Image taken from: http: //library. thinkquest. org/C 0113863/bios. shtml
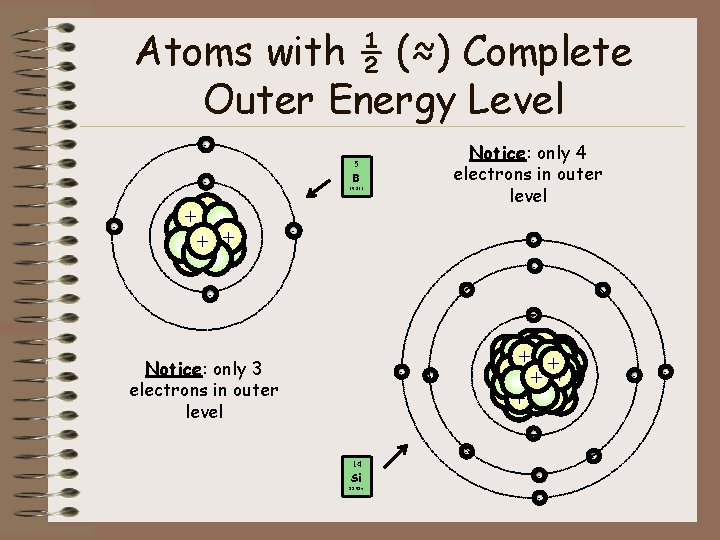
Atoms with ½ (≈) Complete Outer Energy Level Notice: only 4 electrons in outer level - - - 5 B 10. 811 ++ + - - Notice: only 3 electrons in outer level - + +++ + ++ - - 14 Si - 28. 086 - -
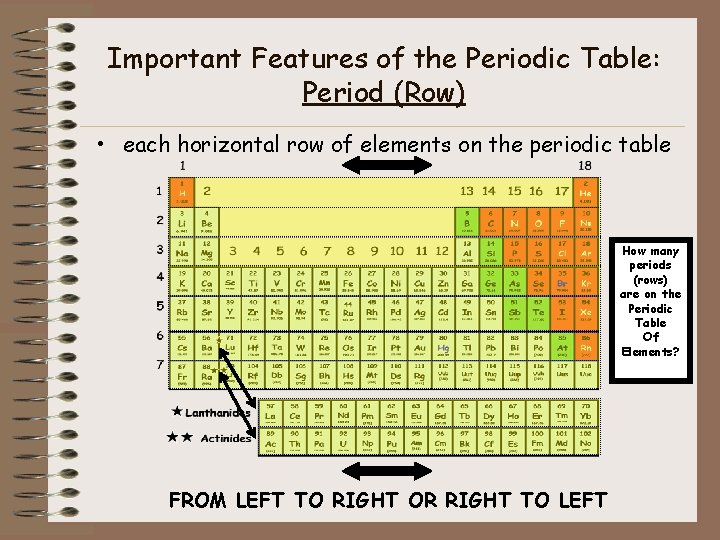
Important Features of the Periodic Table: Period (Row) • each horizontal row of elements on the periodic table How many periods (rows) are on the Periodic Table Of Elements? FROM LEFT TO RIGHT OR RIGHT TO LEFT
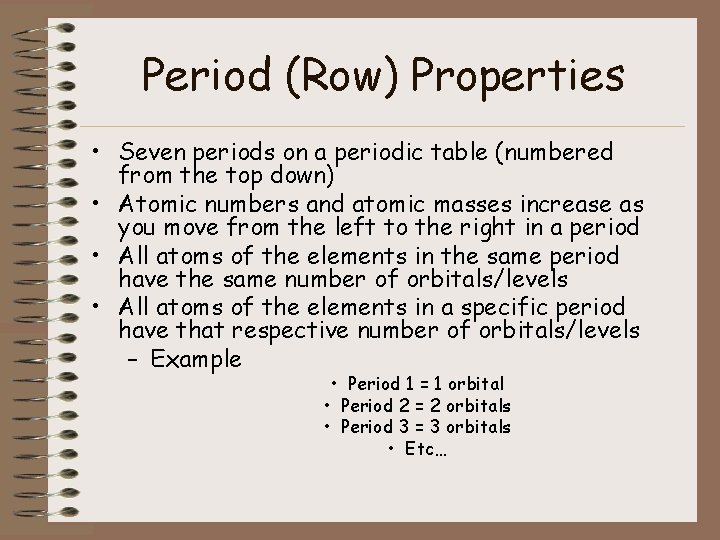
Period (Row) Properties • Seven periods on a periodic table (numbered from the top down) • Atomic numbers and atomic masses increase as you move from the left to the right in a period • All atoms of the elements in the same period have the same number of orbitals/levels • All atoms of the elements in a specific period have that respective number of orbitals/levels – Example • Period 1 = 1 orbital • Period 2 = 2 orbitals • Period 3 = 3 orbitals • Etc…
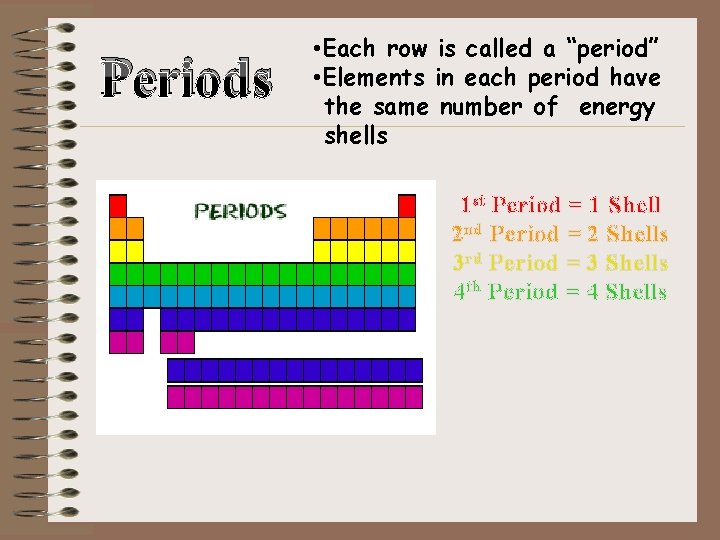
Periods • Each row is called a “period” • Elements in each period have the same number of energy shells 1 st Period = 1 Shell 2 nd Period = 2 Shells 3 rd Period = 3 Shells 4 th Period = 4 Shells
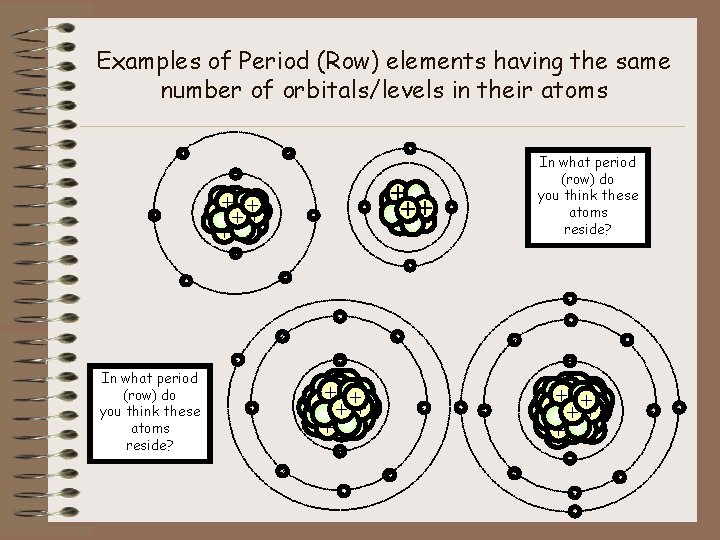
Examples of Period (Row) elements having the same number of orbitals/levels in their atoms - - - In what period (row) do you think these atoms reside? - + ++ + + - - - + ++ + - - - In what period (row) do you think these atoms reside? - - - +++ + ++ + +++ ++ - - -
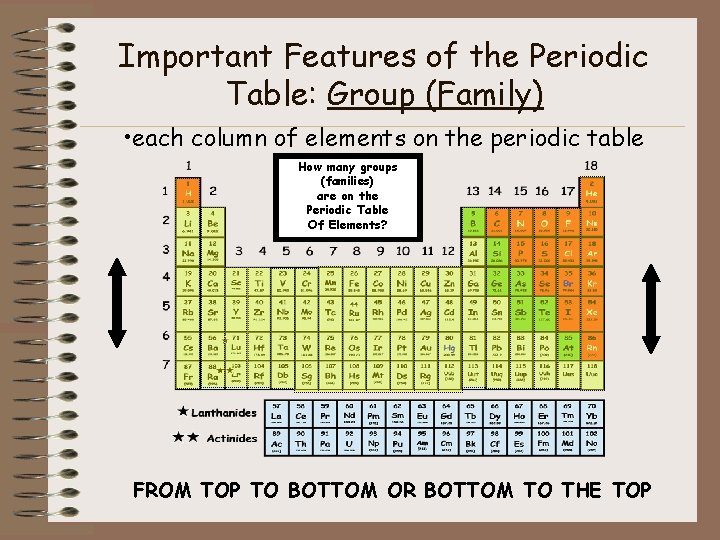
Important Features of the Periodic Table: Group (Family) • each column of elements on the periodic table How many groups (families) are on the Periodic Table Of Elements? FROM TOP TO BOTTOM OR BOTTOM TO THE TOP

Group (Family) Properties • Eighteen groups on the periodic table (numbered from left to right) • Atomic numbers and atomic masses increase as you move from the top down in a group (family) • Atoms of elements in the same group have the same number of electrons in the outer orbital/levels of their atoms (known as valence electrons) – Exceptions: • Transition elements (3 -12) • Hydrogen (has no neutron) • Helium (actually has 2 valence electrons) • Elements in groups usually have similar physical and chemical properties
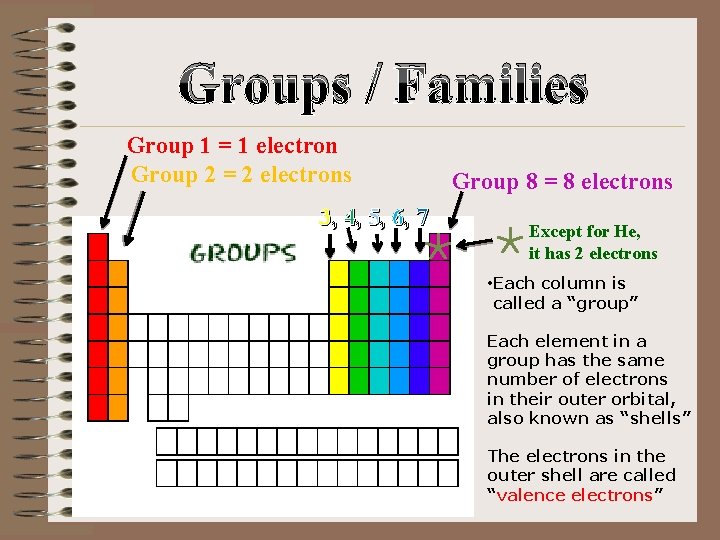
Groups / Families Group 1 = 1 electron Group 2 = 2 electrons 3, 4, 5, 6, 7 Group 8 = 8 electrons Except for He, it has 2 electrons • Each column is called a “group” Each element in a group has the same number of electrons in their outer orbital, also known as “shells” The electrons in the outer shell are called “valence electrons”
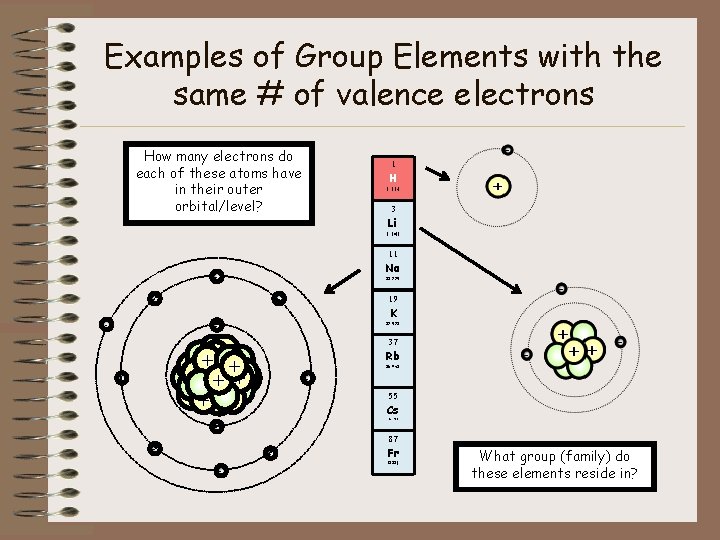
Examples of Group Elements with the same # of valence electrons How many electrons do each of these atoms have in their outer orbital/level? 1 H 1. 008 3 Li 6. 941 11 Na - 22. 990 - 19 - K - 39. 098 37 ++ + + + ++ + - Rb 85. 468 55 Cs 132. 905 87 - - Fr (223) What group (family) do these elements reside in?
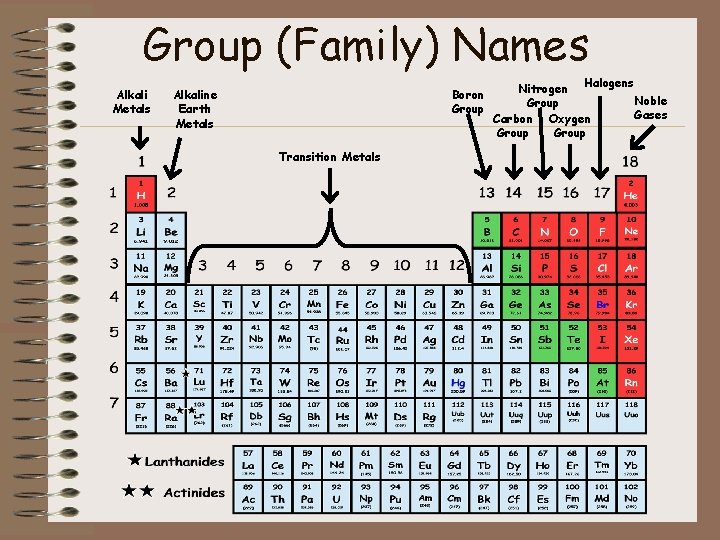
Group (Family) Names Alkali Metals Alkaline Earth Metals Boron Group Transition Metals Nitrogen Halogens Noble Group Gases Carbon Oxygen Group
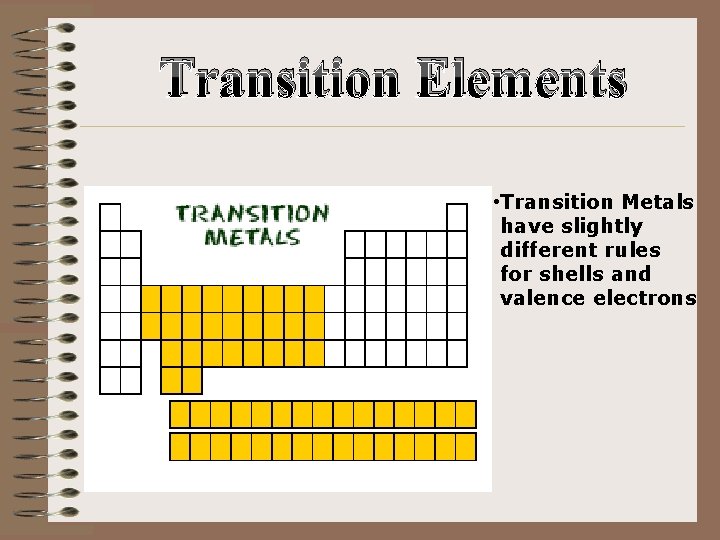
Transition Elements • Transition Metals have slightly different rules for shells and valence electrons

Transition Elements This is something you will learn about in High School Chemistry. They are all metals which are harder than alkali metals and less reactive with water. Their name denotes their central location on the periodic table. Here you find some well known elements like iron, copper, nickel, silver and gold. Because some are noncorrosive they are used in implant devices.
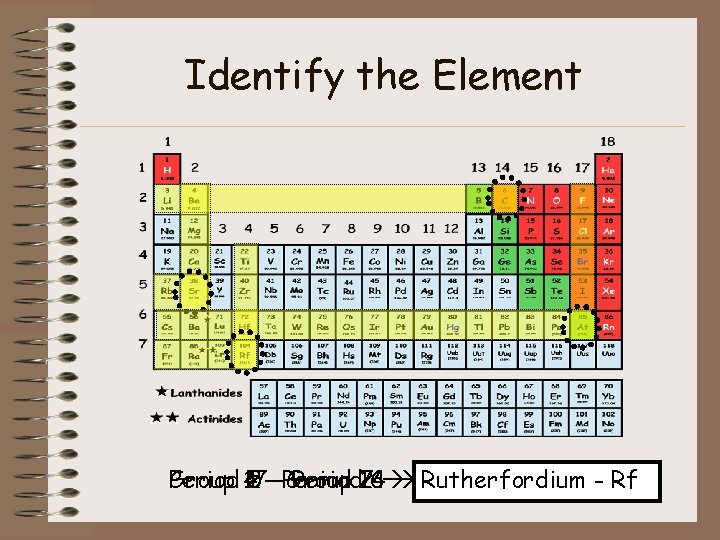
Identify the Element Period 4 Group 17 2 –––Period 5 Group Period 7 14 2 6 Rutherfordium Carbon - C Strontium Astatine - At - Sr- Rf
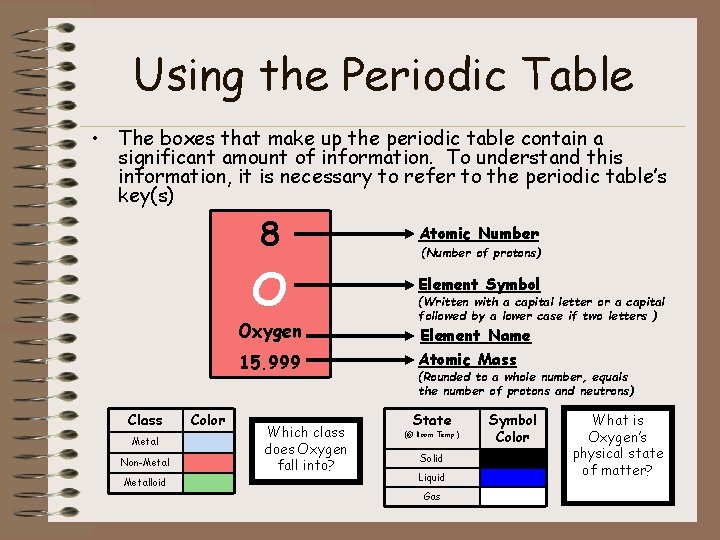
Using the Periodic Table • The boxes that make up the periodic table contain a significant amount of information. To understand this information, it is necessary to refer to the periodic table’s key(s) 8 Atomic Number O Element Symbol Oxygen 15. 999 Class Metal Non-Metalloid Color Which class does Oxygen fall into? (Number of protons) (Written with a capital letter or a capital followed by a lower case if two letters ) Element Name Atomic Mass (Rounded to a whole number, equals the number of protons and neutrons) State (@ Room Temp. ) Solid Liquid Gas Symbol Color What is Oxygen’s physical state of matter?
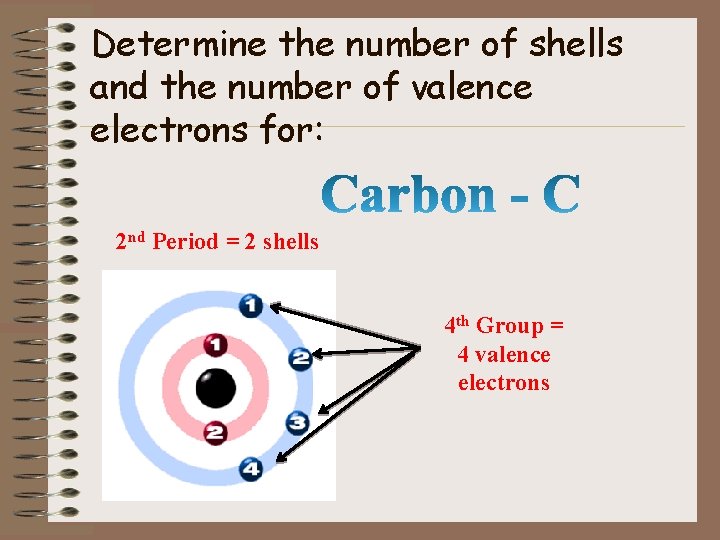
Determine the number of shells and the number of valence electrons for: 2 nd Period = 2 shells 4 th Group = 4 valence electrons
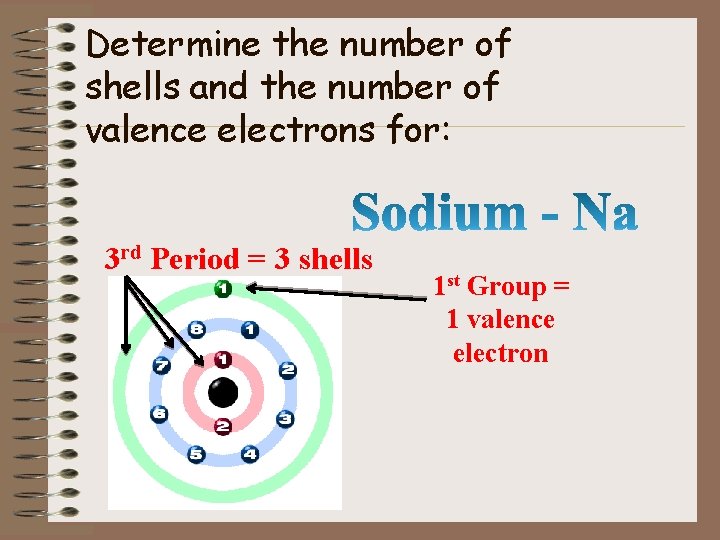
Determine the number of shells and the number of valence electrons for: 3 rd Period = 3 shells 1 st Group = 1 valence electron

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ?

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ? Neon 2 nd Period = 2 shells 8 th Group = 8 valence electrons

Write your answers on your handout. Name the element. Number of shells ? Valence electrons ?

Write your answers on your handout. Name the element. Hydrogen Number of shells ? 1 st Period = 1 shell Valence electrons ? 1 st Group = 1 valence electron
- Slides: 35