PERIODIC TRENDS Atomic Radius Atomic radius is the
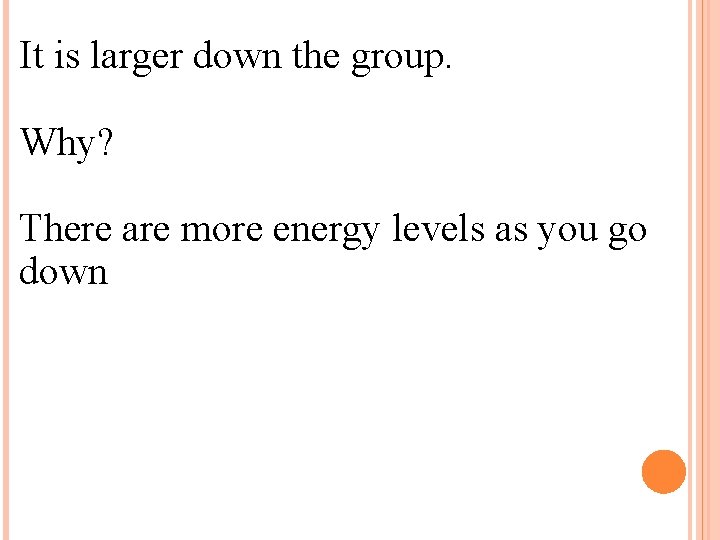















- Slides: 37

PERIODIC TRENDS

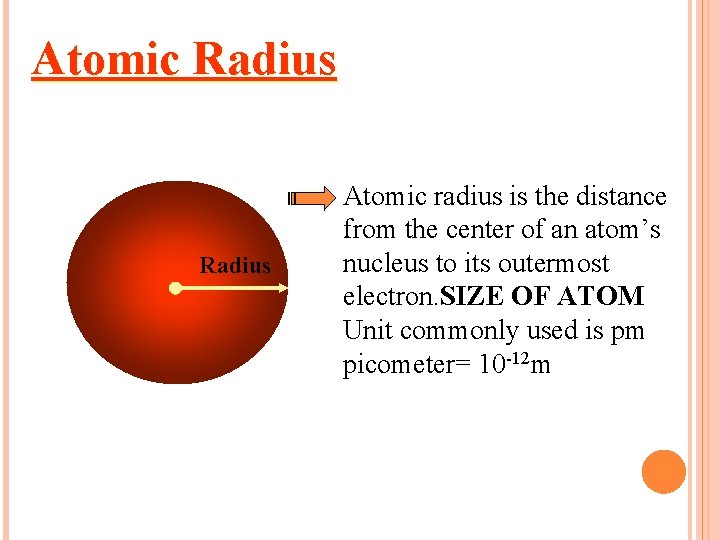
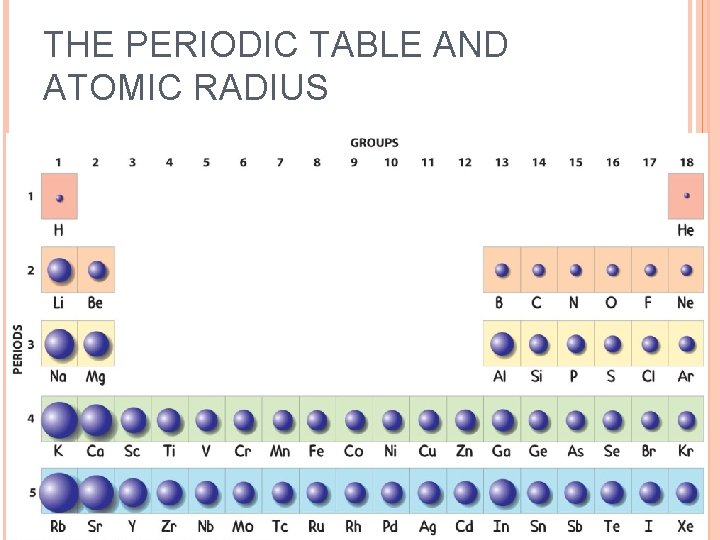
Atomic Radius Atomic radius is the distance from the center of an atom’s nucleus to its outermost electron. SIZE OF ATOM Unit commonly used is pm picometer= 10 -12 m


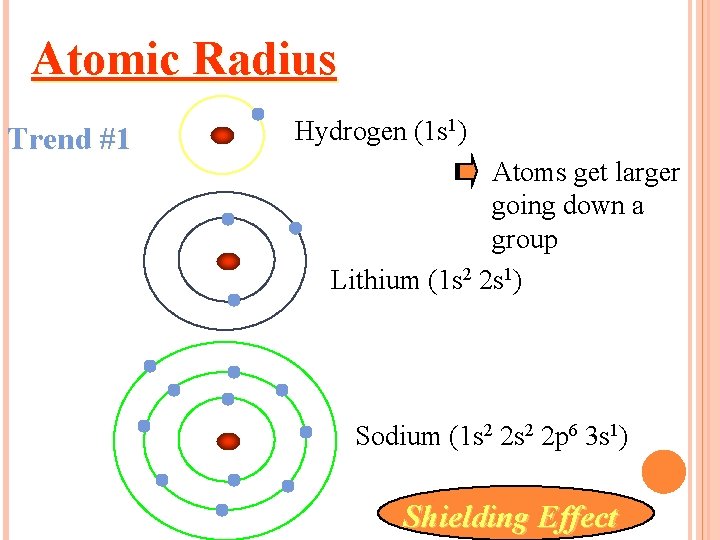
Atomic Radius Trend #1 Hydrogen (1 s 1) Atoms get larger going down a group Lithium (1 s 2 2 s 1) Sodium (1 s 2 2 p 6 3 s 1) Shielding Effect
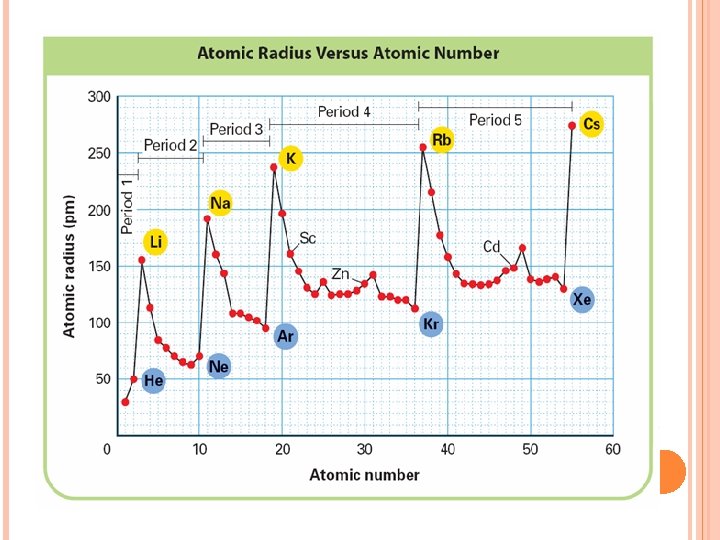
It is larger down the group. Why? There are more energy levels as you go down

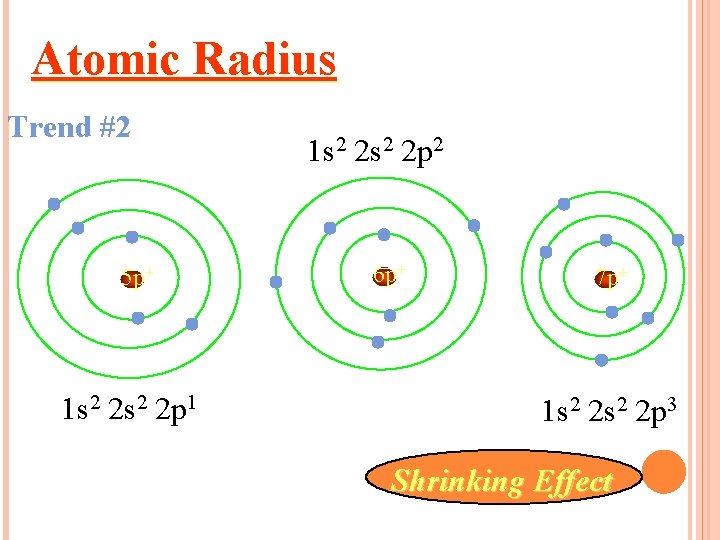
Atomic Radius Trend #2 5 p+ 1 s 2 2 p 1 1 s 2 2 p 2 6 p+ 7 p+ 1 s 2 2 p 3 Shrinking Effect


Atoms decrease in size as you go across a period Why? More protons in the nucleus higher electrical force pulls electrons closer to nucleus.


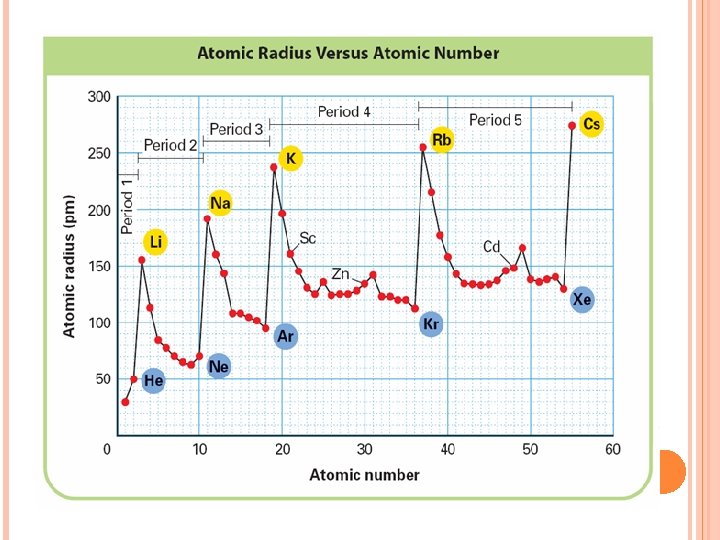
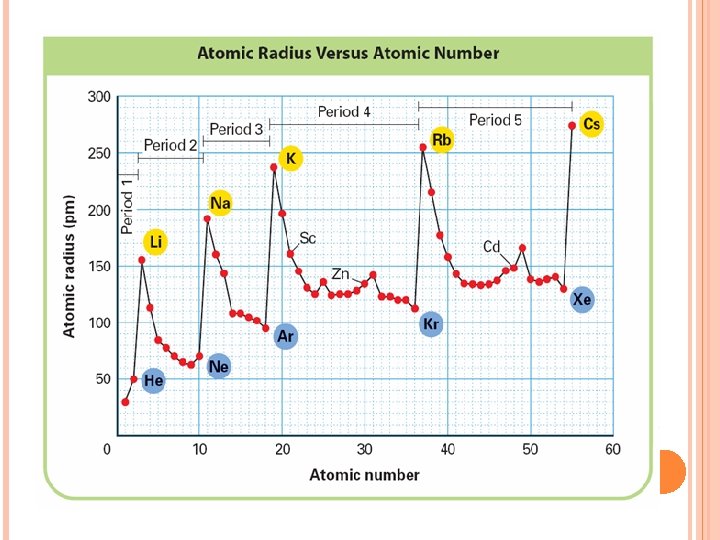
THE PERIODIC TABLE AND ATOMIC RADIUS

EXAMPLE: Which is larger: a lithium atom or a fluorine atom? A lithium atom

EXAMPLE: Which is larger: an arsenic atom or a sulfur atom? An arsenic atom

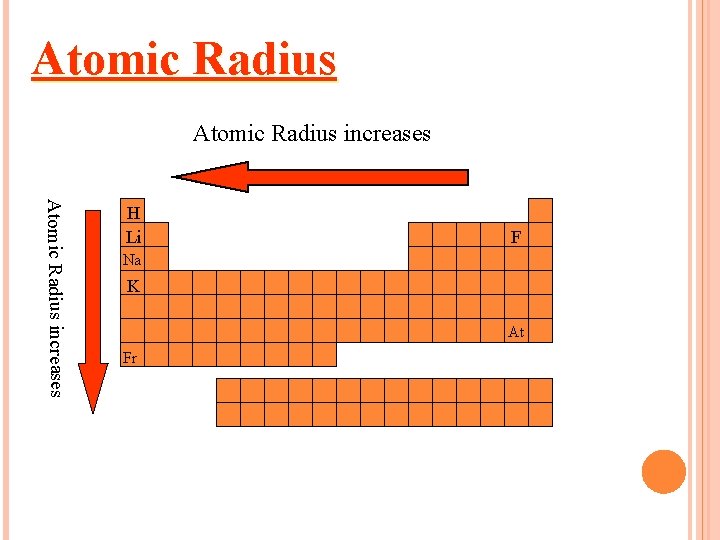
Atomic Radius increases H Li F Na K At Fr

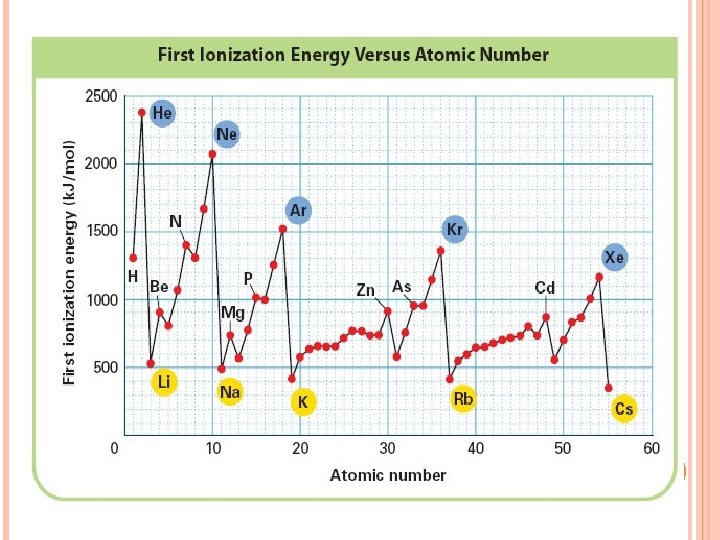
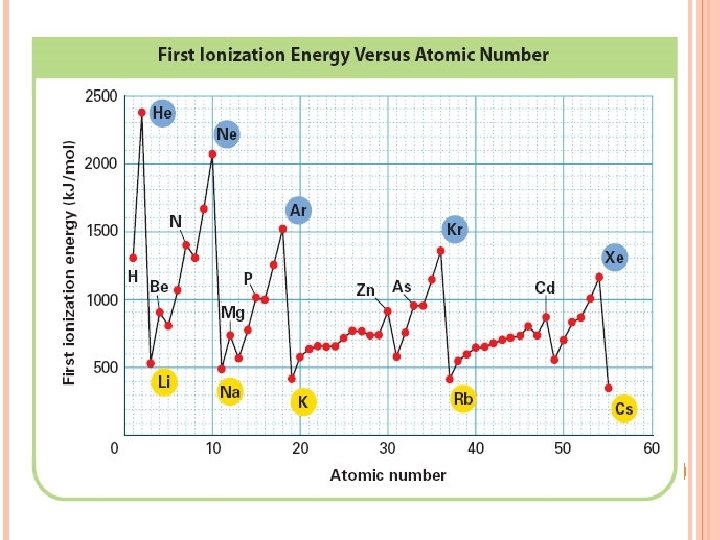
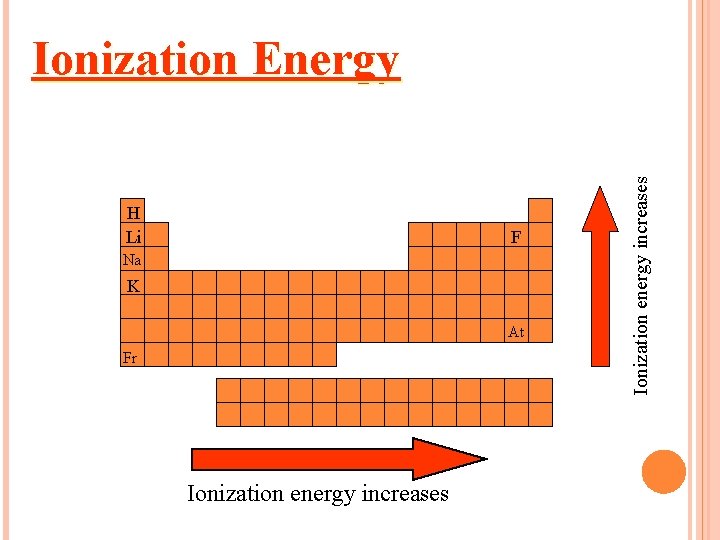
Ionization Energy needed to remove one of atom’s electrons from its outermost shell Reflection of how strongly an atom holds onto its outermost electron. Atoms with high ionization energies hold onto their electrons very tightly. Atoms with low ionization energies are more likely to lose one or more of their outermost electron.


HOW DOES IONIZATION ENERGY CHANGE DOWN A GROUP? The first ionization energy decreases as you move down a group. Why? The size of the atom increases. Electron is farther from the nucleus.


HOW DOES IONIZATION ENERGY CHANGE ACROSS A PERIOD? The first ionization energy increases as you move from left to right across a period. Why? Nuclear charge increases – more protons. Attraction of the electron to the nucleus increases.

H Li F Na K At Fr Ionization energy increases Ionization Energy

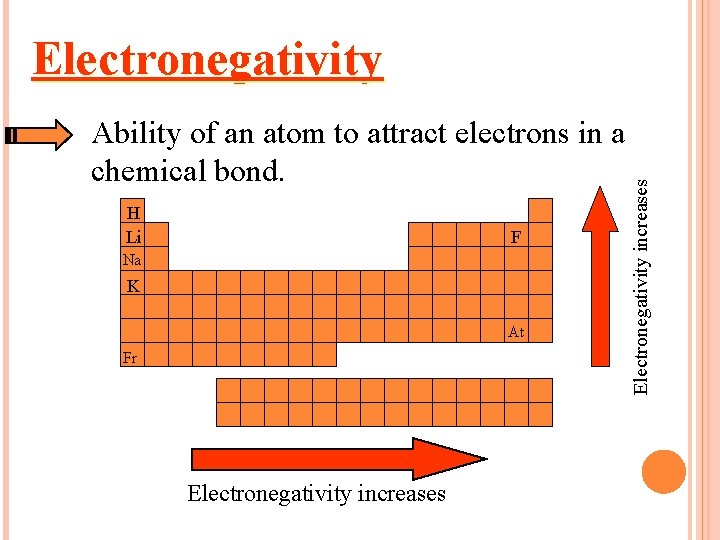
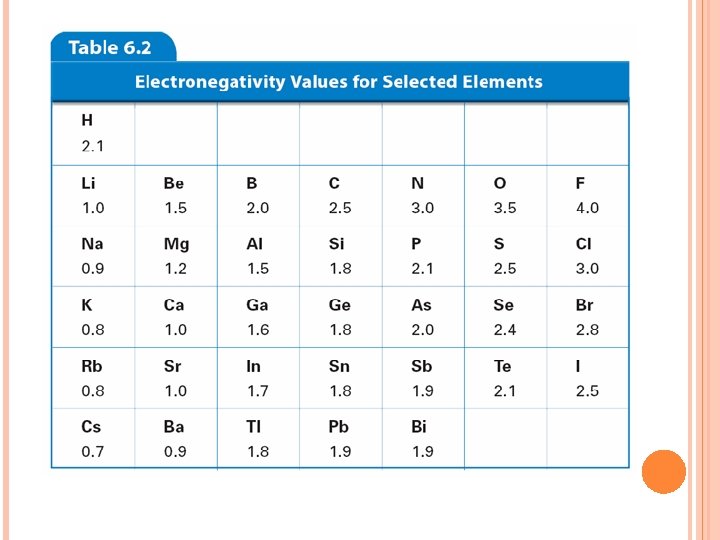
Ability of an atom to attract electrons in a chemical bond. H Li F Na K At Fr Electronegativity increases Electronegativity


ION—CHARGED ATOM Positive ion ---removal of electron (cation) Negative ion--- addition of electron (anion)

IONIC RADIUS The size of the ion— (charged atom)

IONIC SIZE Metallic elements easily lose electrons. Non-metals more readily gain electrons. How does losing or gaining an electron effect the size of the atom (ion) ?

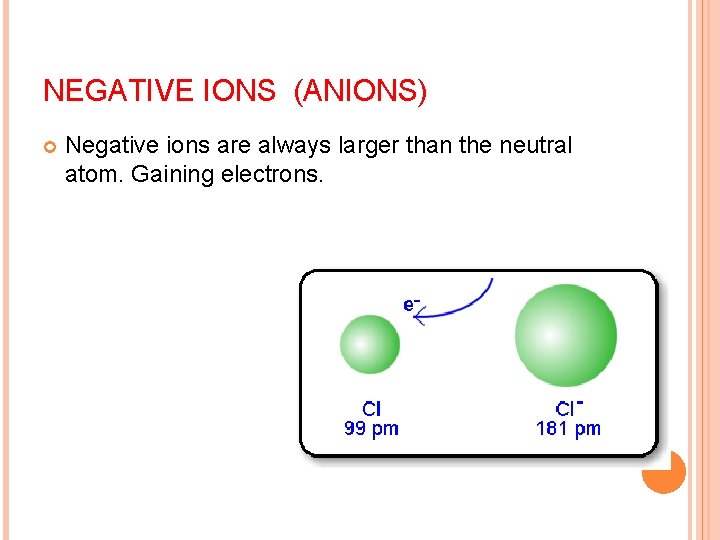
IONIC RADIUS Anion, (negative ion) Gains e its size increases, since the nuclear charge(protons)remains the same but the additional electron(s) enlarges the electron cloud.

NEGATIVE IONS (ANIONS) Negative ions are always larger than the neutral atom. Gaining electrons.

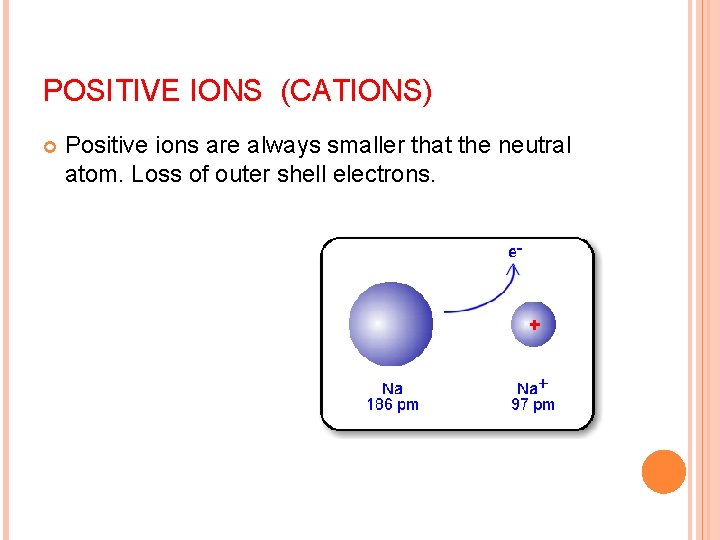
IONIC RADIUS Cation (positive ion) Loses e smaller than neutral atom, since removing one or more e- makes the cloud shrink, the nuclear charge remains the same

POSITIVE IONS (CATIONS) Positive ions are always smaller that the neutral atom. Loss of outer shell electrons.

ION SIZE TRENDS IN PERIODS. Going from left to right there is a decrease in size of positive ions. Starting with group 5, there is sharp increase followed by a decrease in the size of the anion as you move from left to right.

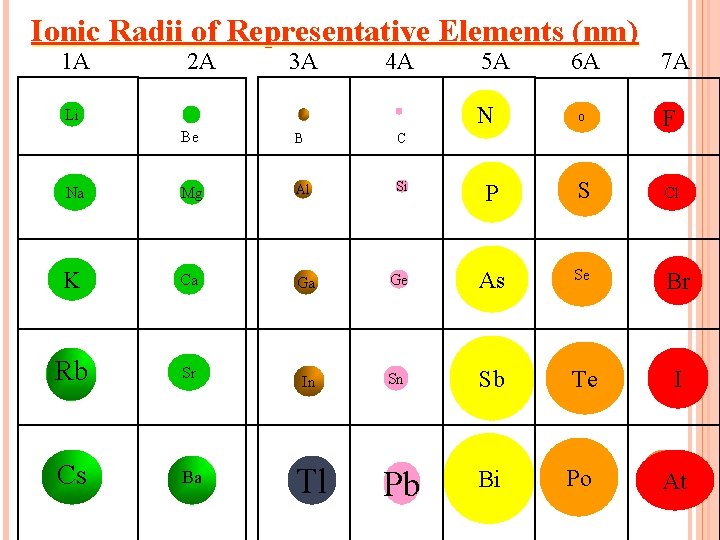
ION SIZE TRENDS IN COLUMNS. Ion size increases as you move down a column for both positive and negative ions

Ionic Radii of Representative Elements (nm) 1 A 2 A 3 A 4 A 5 A N Li 6 A 7 A O F Be B C Na Mg Al Si P S Cl K Ca Ga Ge As Se Br Rb Sr In Sn Sb Te I Cs Ba Pb Bi Po At Tl

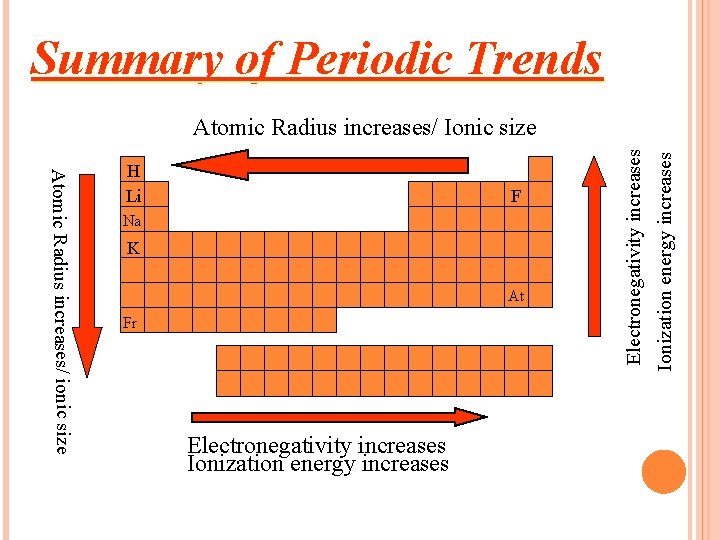
Summary of Periodic Trends F Na K At Fr Electronegativity increases Ionization energy increases Atomic Radius increases/ ionic size H Li Electronegativity increases Atomic Radius increases/ Ionic size

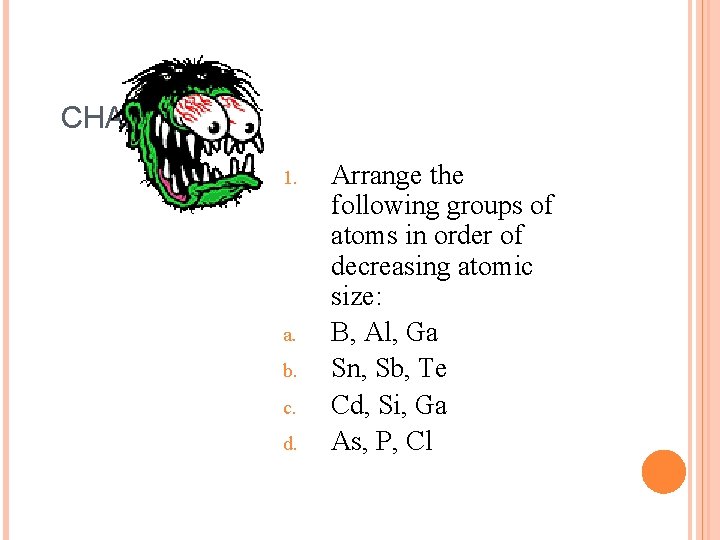
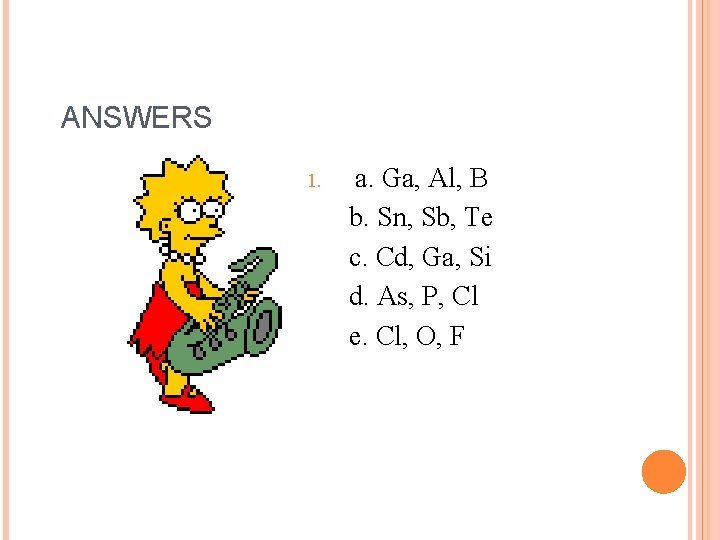
CHALLENGE 1. a. b. c. d. Arrange the following groups of atoms in order of decreasing atomic size: B, Al, Ga Sn, Sb, Te Cd, Si, Ga As, P, Cl

ANSWERS 1. a. Ga, Al, B b. Sn, Sb, Te c. Cd, Ga, Si d. As, P, Cl e. Cl, O, F

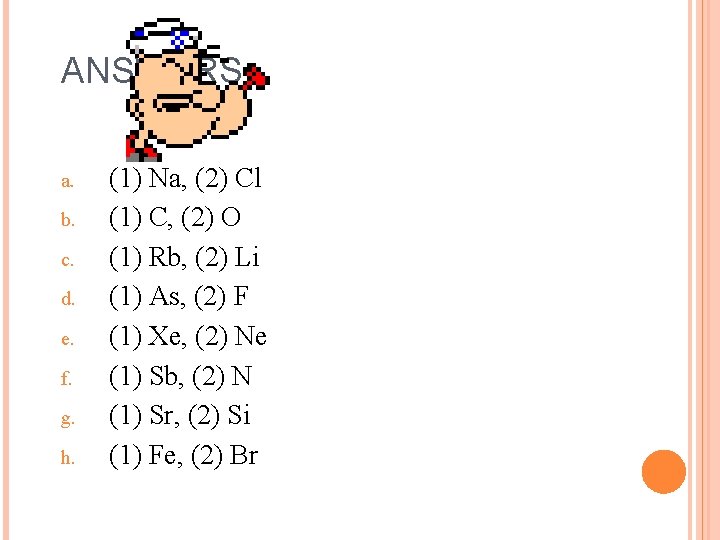
CHALLENGE 2. For each of the following pairs, predict which element has (1) the larger radius, and (2) the larger ionization energy: a. Na & Cl b. C&O c. Li & Rb d. As & F e. Ne & Xe f. N & Sb g. Sr & Si h. Fe & Br

ANSWERS a. b. c. d. e. f. g. h. (1) Na, (2) Cl (1) C, (2) O (1) Rb, (2) Li (1) As, (2) F (1) Xe, (2) Ne (1) Sb, (2) N (1) Sr, (2) Si (1) Fe, (2) Br

CHALLENGE 3. List the following ions in order of increasing ionic radius: N 3 -, Na+, F-, Mg 2+, O 24. Indicate which one of the two species in each of the following pairs is smaller: a. Cl or Clb. Na or Na+ c. O 2 - or S 2 d. Mg 2+ or Al 3+ e. Au+ or Au 3+