Periodic Trends Types of Periodic Trends 5 Periodic











- Slides: 23

> Periodic Trends Types of Periodic Trends 5 Periodic Trends 1. Atomic Radii (AR) 2. Ionization Energy (IE) 3. Electronegativity (EN) 4. Ionic Radii (IR) 5. Metallic Charateristic (MC) Slide 1 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Columbic Force (CF) (explanation for Periodic Trends) Opposite Charged Particles – Attract Same Charged Particles – Repel F = (Q 1*Q 2)/r Q 1 – charge on particle 1 (nucleus “+” charge) Q 2 – charge on particle 2 (valence e, “-” charge) r – distance between particles Slide 2 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Columbic Force – Periodic Trend for Columbic Force (CF) Down a Group: Trend: Reason: Across a Period: Trend: Reason: Slide 3 of 31 © Copyright Pearson Prentice Hall

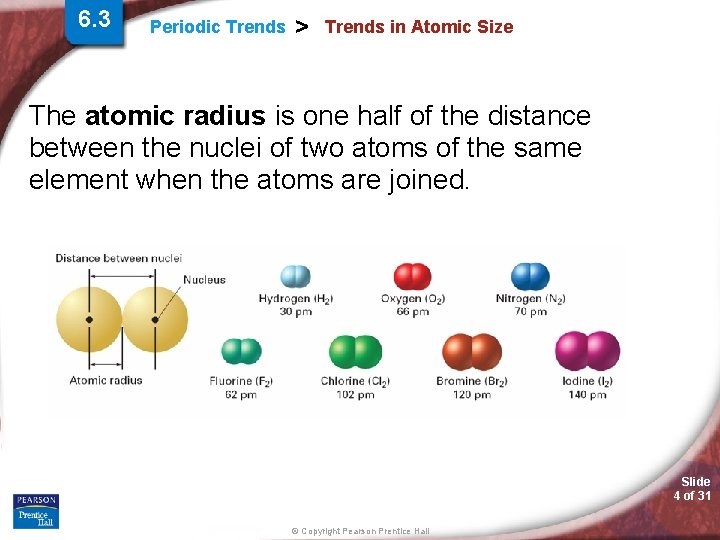
6. 3 Periodic Trends > Trends in Atomic Size The atomic radius is one half of the distance between the nuclei of two atoms of the same element when the atoms are joined. Slide 4 of 31 © Copyright Pearson Prentice Hall

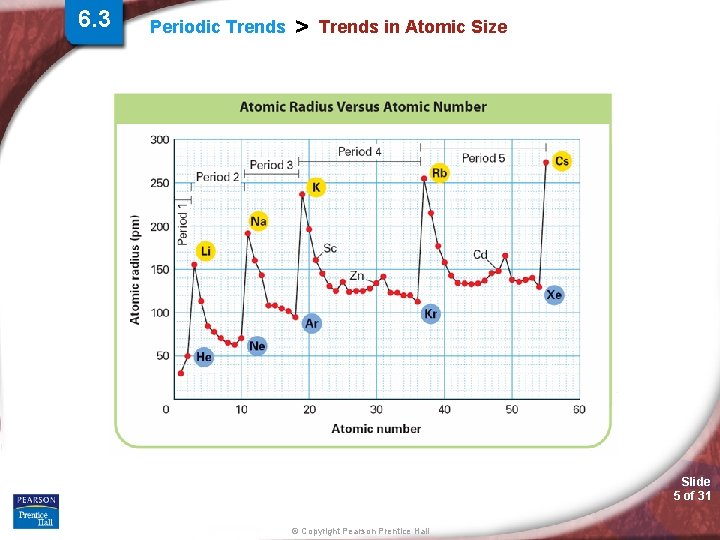
6. 3 Periodic Trends > Trends in Atomic Size Slide 5 of 31 © Copyright Pearson Prentice Hall

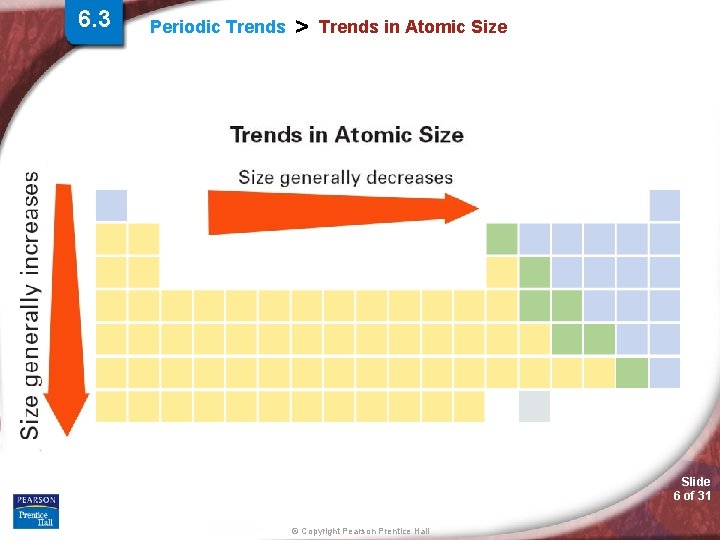
6. 3 Periodic Trends > Trends in Atomic Size Slide 6 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Atomic Radii (AR) Periodic Trend for Atomic Radii (AR) Down a Group: Trend: Reason: Across a Period: Trend: Reason: Slide 7 of 31 © Copyright Pearson Prentice Hall

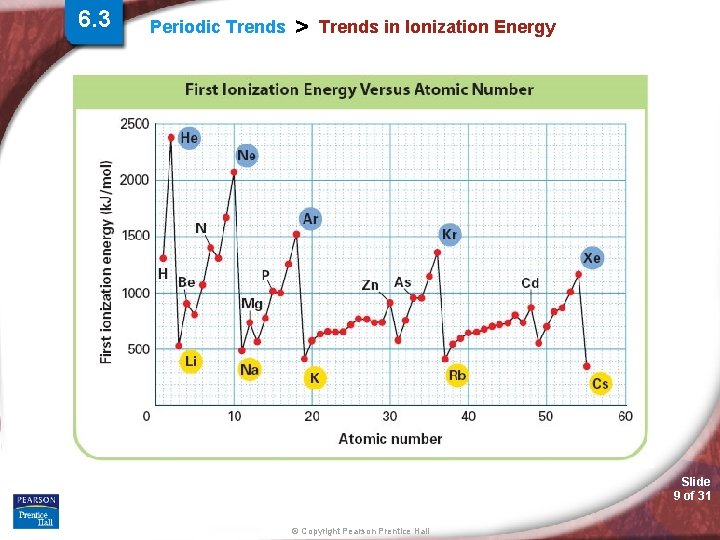
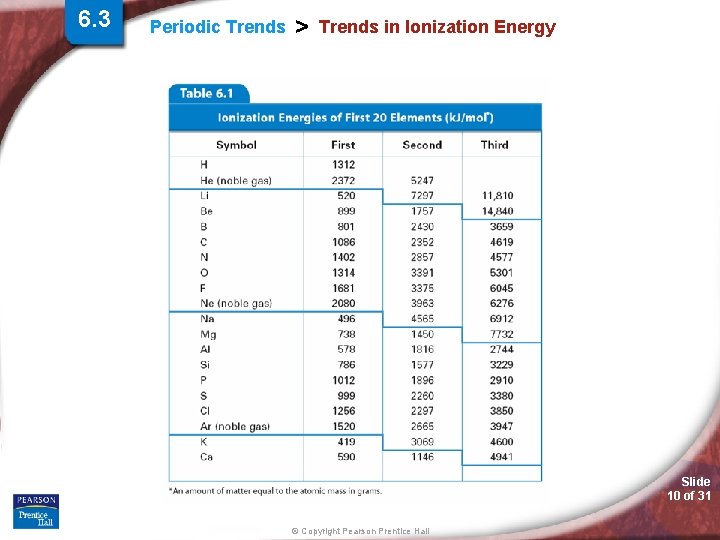
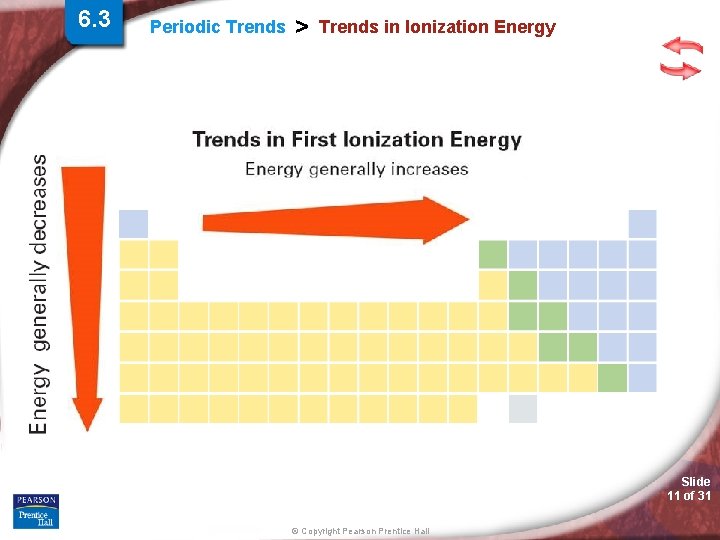
6. 3 Periodic Trends > Trends in Ionization Energy (IE) - The energy required to remove the first valence electron from an atom (atom is in the gas phase). • What is holding the valence electron to the atom? • Valence electron tightly held or loosely? Slide 8 of 31 © Copyright Pearson Prentice Hall

6. 3 Periodic Trends > Trends in Ionization Energy Slide 9 of 31 © Copyright Pearson Prentice Hall

6. 3 Periodic Trends > Trends in Ionization Energy Slide 10 of 31 © Copyright Pearson Prentice Hall

6. 3 Periodic Trends > Trends in Ionization Energy Slide 11 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Ionization Energy (IE) Periodic Trend for Ionization Energy (IE) Down a Group: Trend: Reason: Across a Period: Trend: Reason: Slide 12 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Ionization Energy (IE) Periodic Trends Two Exception to 1 st Ionization Energy (IE) (Across the Period): 1. Be B Trend: Reason: 2. N O Trend: Reason: Slide 13 of 31 © Copyright Pearson Prentice Hall

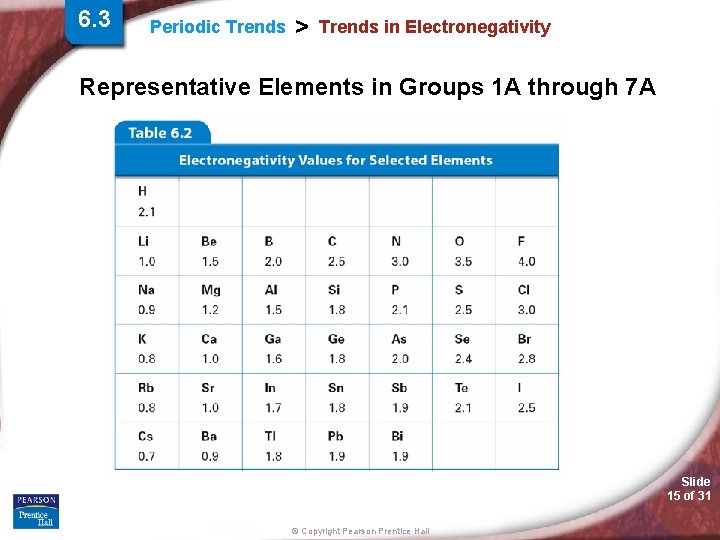
6. 3 Periodic Trends > Trends in Electronegativity • Trends in Electronegativity is the ability of an atom, in a chemical bond, to attract the shared valence electrons to itself. (i. e. the shared valence electrons are physically closer to the higher EN value atom than the other atom in the chemical bond). • What EN really means? • Fluorine? Slide 14 of 31 © Copyright Pearson Prentice Hall

6. 3 Periodic Trends > Trends in Electronegativity Representative Elements in Groups 1 A through 7 A Slide 15 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Electronegativity (EN) Periodic Trend for Electronegativity (EN) Down a Group: Trend: Reason: Across a Period: Trend: Reason: Slide 16 of 31 © Copyright Pearson Prentice Hall

6. 3 Periodic Trends > Ions An ion is an atom or group of atoms that has a positive or negative charge. • A cation is an ion with a positive charge. • An anion is an ion with a negative charge. Slide 17 of 31 © Copyright Pearson Prentice Hall

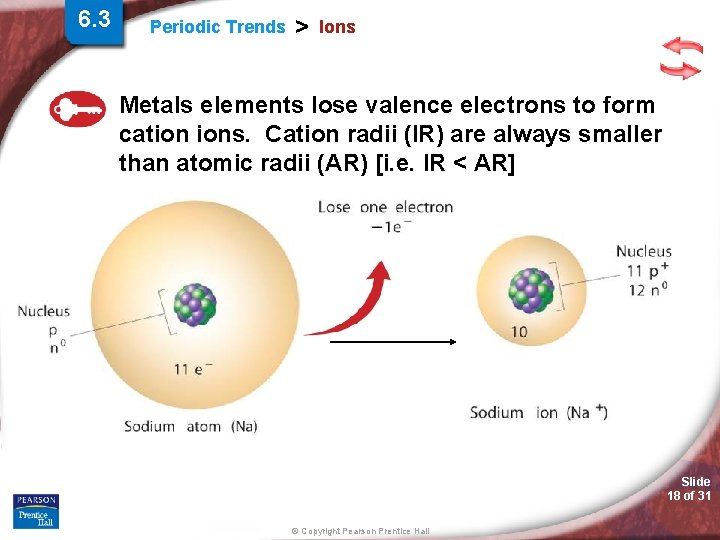
6. 3 Periodic Trends > Ions Metals elements lose valence electrons to form cation ions. Cation radii (IR) are always smaller than atomic radii (AR) [i. e. IR < AR] Slide 18 of 31 © Copyright Pearson Prentice Hall

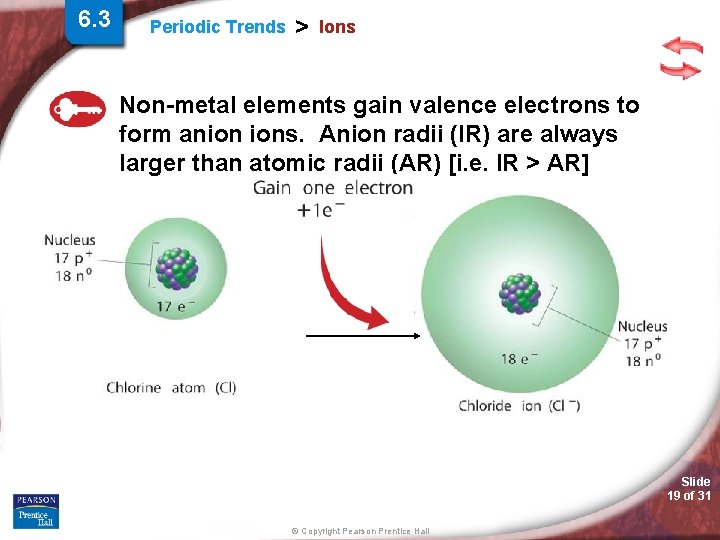
6. 3 Periodic Trends > Ions Non-metal elements gain valence electrons to form anion ions. Anion radii (IR) are always larger than atomic radii (AR) [i. e. IR > AR] Slide 19 of 31 © Copyright Pearson Prentice Hall

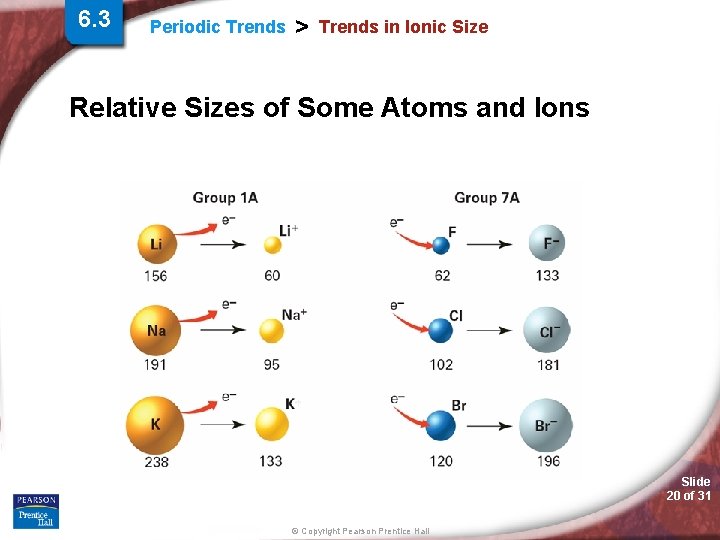
6. 3 Periodic Trends > Trends in Ionic Size Relative Sizes of Some Atoms and Ions Slide 20 of 31 © Copyright Pearson Prentice Hall

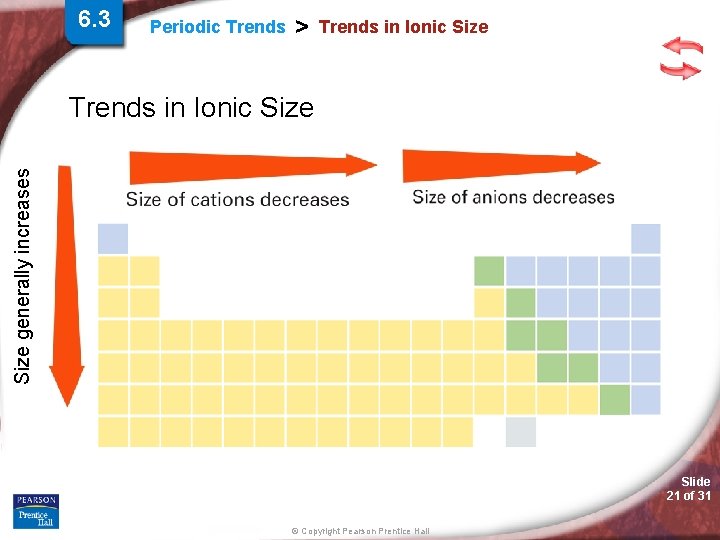
6. 3 Periodic Trends > Trends in Ionic Size generally increases Trends in Ionic Size Slide 21 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Ionic Radii Periodic Trend for Ionic Radii (IR) Down a Group: Trend: Reason: Across a Period: Metals large small, then Non-metal large small (Caution: Are not looking at same charge ion when across period) Metals: IR vs. AR : Reason: Non-metals: IR vs. AR : Reason: Slide 22 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Metallic Characteristics Periodic Trend Definition of Metals Composed of cations in a “sea” of free flowing valence electrons. • Properties – Good Electrical Conductor • Properties – Good Heat Conductor • Properties – Malleable & Ductile • Periodic Trends (Metallic Characterstic) Slide 23 of 31 © Copyright Pearson Prentice Hall