Periodic Trends Notes Periodic Trends Notes The periodic




























- Slides: 42

Periodic Trends Notes

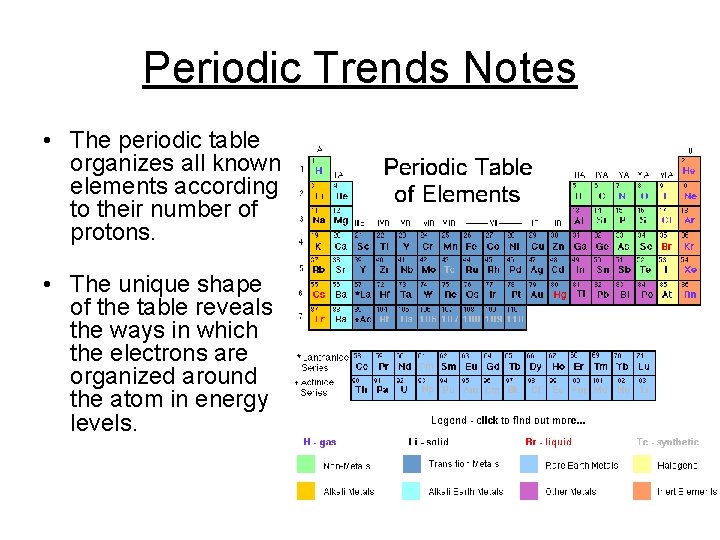
Periodic Trends Notes • The periodic table organizes all known elements according to their number of protons. • The unique shape of the table reveals the ways in which the electrons are organized around the atom in energy levels.

Periodic Trends Notes • The structure of an element (especially the number of protons and the number of electrons) will determine the properties of the element (how it will appear, how it will act, etc. ) • The periodic table can be broken into regions where all the elements in a region have very similar properties. These regions (and subregions) are commonly given names:


Periodic Trends Notes • 1. The largest region is the Metals. On page 120, everything in red is a metal, and they all have certain characteristics in common. They conduct heat and electricity very easily, they are lustrous (shiny) and malleable (can be bent, flattened, etc).

Periodic Trends Notes • 2. The second largest region is the Nonmetals. These elements (green and brown on page 120) are generally poor conductors of heat and electricity, and when solid are brittle (can be broken or shattered).

Periodic Trends Notes • 3. Another region is the Metalloids. These elements (orange on page 120) have characteristics of metals and nonmetals, which vary with the element. (above: selenium, below: silicon)

Periodic Trends Notes • 4. Noble gases are located on the far right (purple on page 120) and rarely (some never) react with other elements in chemical reactions. • This is because they have enough electrons to fill up the p orbital, which makes them very stable.

Periodic Trends Notes • **Octet Rule**--The “goal” of most atoms (except H, Li and Be) is to have an octet or group of 8 electrons in their valence energy level. • They may accomplish this by either giving electrons away or taking them. • Metals generally give electrons, nonmetals take them from other atoms.

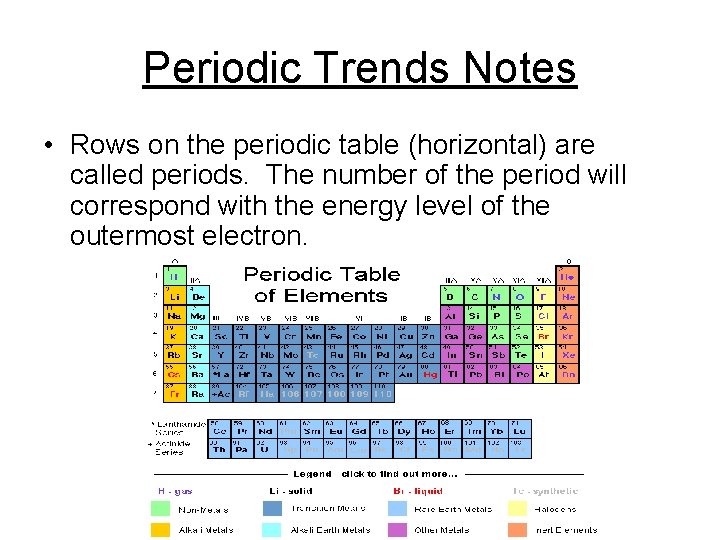
Periodic Trends Notes • Rows on the periodic table (horizontal) are called periods. The number of the period will correspond with the energy level of the outermost electron.

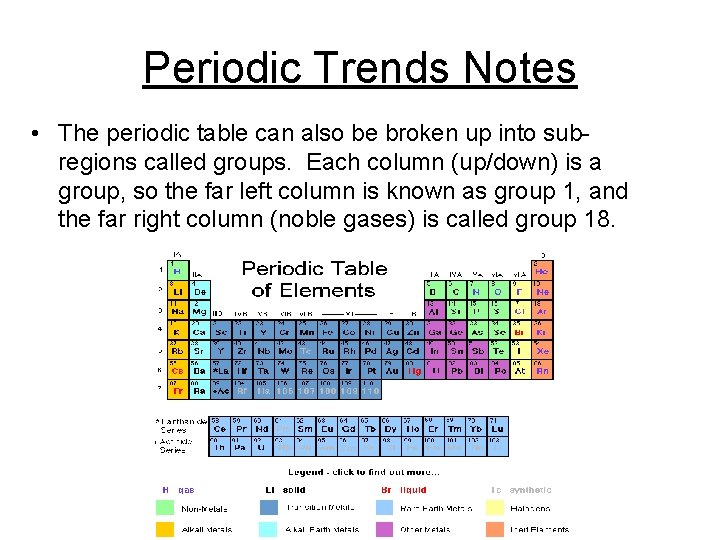
Periodic Trends Notes • The periodic table can also be broken up into subregions called groups. Each column (up/down) is a group, so the far left column is known as group 1, and the far right column (noble gases) is called group 18.

Periodic Trends Notes • Typically groups that are next to each other will have similar chemical properties, and the further away two groups are the more different they will be.

Periodic Trends Notes • Some groups have names: 1. Group 1 is called the Alkali Metals. They are soft and highly reactive. This is because they have a single electron in their s orbital, which can be lost very easily in chemical reactions, which will make them more stable.

Periodic Trends Notes • 2. Group 2 is called the Alkaline -Earth Metals. They are less reactive than Alkali Metals because they have two electrons in the s orbital, which is more difficult to lose in order to be stable. They are also harder and more dense (therefore strong). However, they are still very reactive.

Periodic Trends Notes • 3. Groups 3 -12 are called Transition Metals. They widely vary in chemical properties. Among the transition metals are the two rows on the very bottom (f orbitals). The first row is called the Lanthanides, and the second row is called the Actinides.

Periodic Trends Notes • 4. Group 17 is called the Halogens. These are the most reactive non-metals, because they need only one electron to fill their octet.

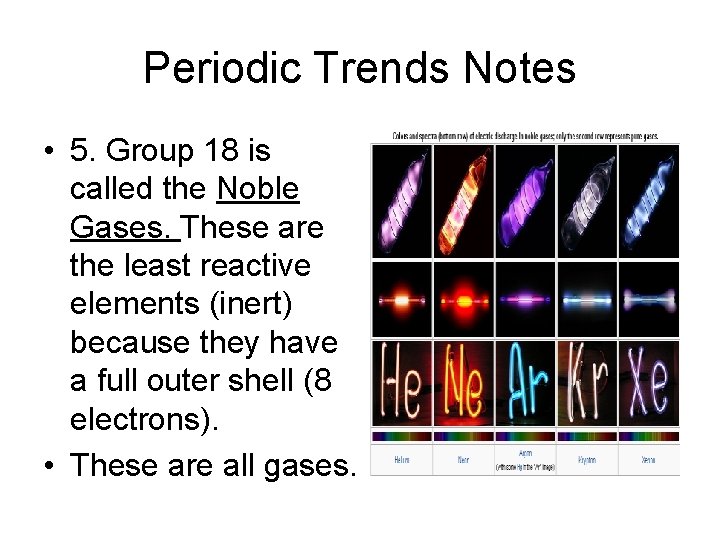
Periodic Trends Notes • 5. Group 18 is called the Noble Gases. These are the least reactive elements (inert) because they have a full outer shell (8 electrons). • These are all gases.

Periodic Trends Notes • 6. Hydrogen is the only element that composes its own region of the periodic table.

Periodic Trends • Periodic Trends – patterns (don’t always hold true) can be seen with our current arrangement of the elements (Moseley) • 1. 2. 3. Trends we’ll be looking at: Atomic Radius Ionization Energy Electronegativity

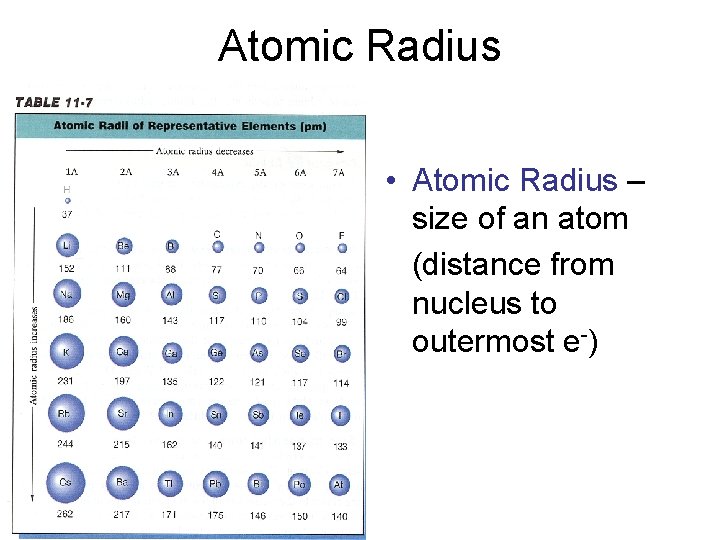
Atomic Radius • Atomic Radius – size of an atom (distance from nucleus to outermost e-)

Atomic Radius Trend • Group Trend – As you go down a column, atomic radius increases As you go down, e- are filled into orbitals that are farther away from the nucleus (attraction not as strong) • Periodic Trend – As you go across a period (L to R), atomic radius decreases As you go L to R, e- are put into the same orbital, but more p+ and e- total (more attraction = smaller size)

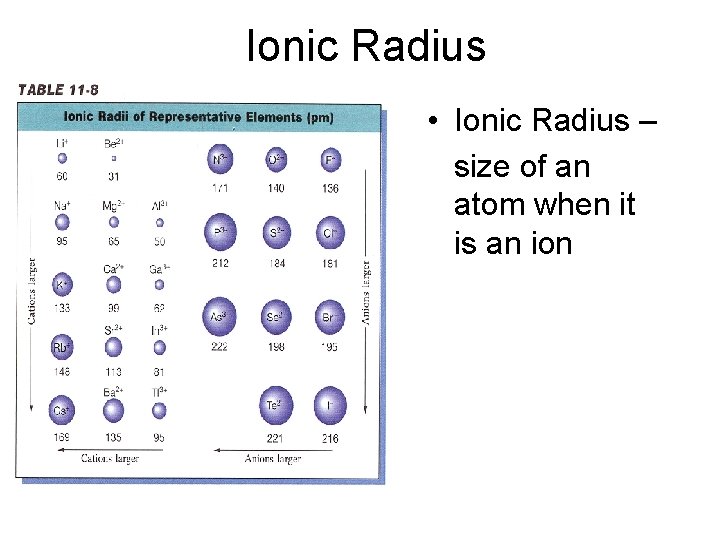
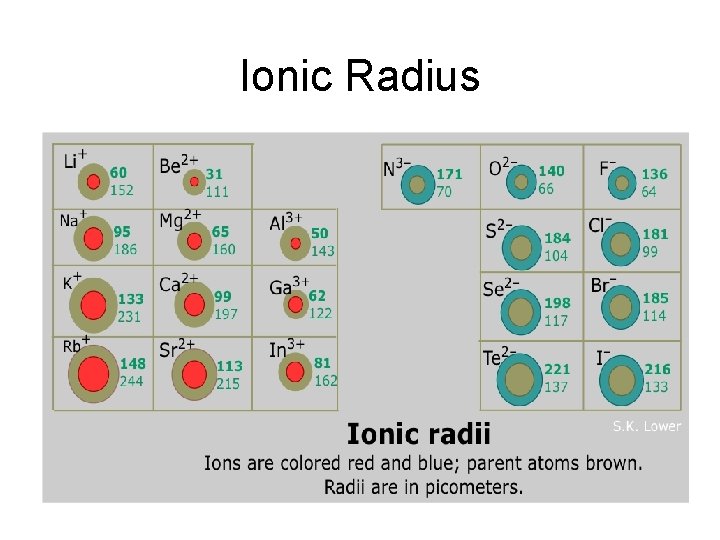
Ionic Radius • Ionic Radius – size of an atom when it is an ion

Ionic Radius Trend Metals – lose e-, which means more p+ than e(more attraction) SO… Cation Radius < Neutral Atomic Radius Nonmetals – gain e-, which means more e- than p+ (not as much attraction) SO… Anion Radius > Neutral Atomic Radius

Ionic Radius Trend • Group Trend – As you go down a column, ionic radius increases • Periodic Trend – As you go across a period (L to R), cation radius decreases, anion radius decreases, too. As you go L to R, cations have more attraction (smaller size because more p+ than e-). The anions have a larger size than the cations, but also decrease L to R because of less attraction (more e- than p+)

Ionic Radius

Ionic Radius How do I remember this? ? ? The more electrons that are lost, the greater the reduction in size. Li+1 Be+2 protons 3 protons 4 electrons 2 Which ion is smaller?

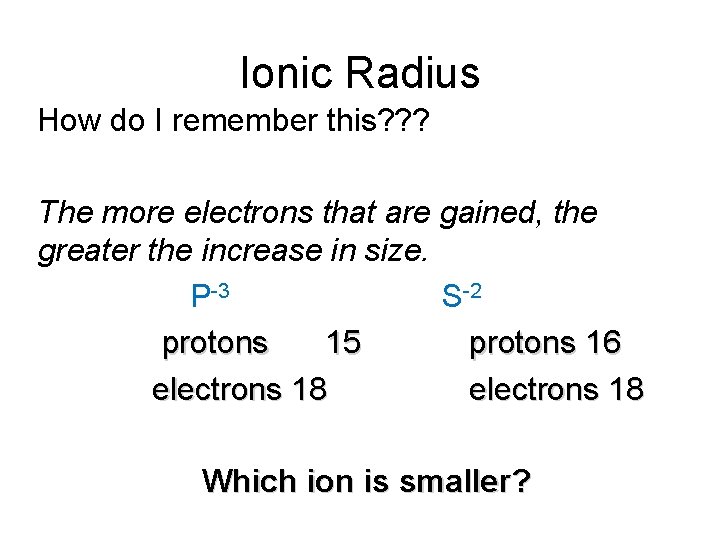
Ionic Radius How do I remember this? ? ? The more electrons that are gained, the greater the increase in size. P-3 S-2 protons 15 protons 16 electrons 18 Which ion is smaller?

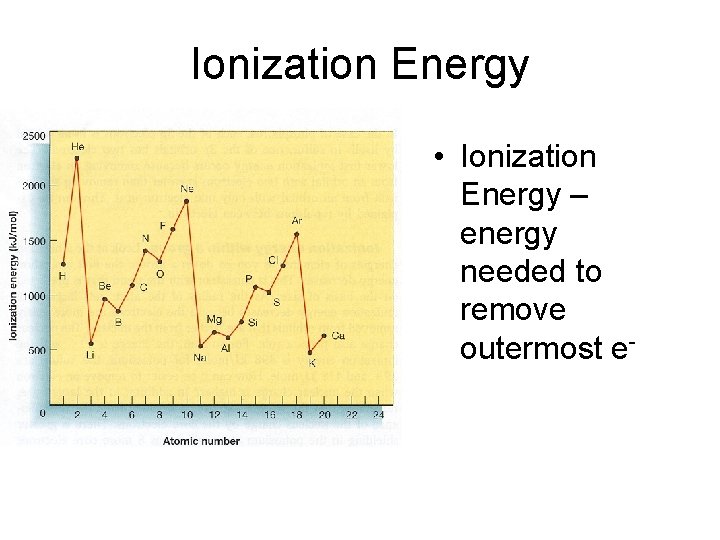
Ionization Energy • Ionization Energy – energy needed to remove outermost e-

Ionization Energy • Group Trend – As you go down a column, ionization energy decreases As you go down, atomic size is increasing (less attraction), so easier to remove an e • Periodic Trend – As you go across a period (L to R), ionization energy increases As you go L to R, atomic size is decreasing (more attraction), so more difficult to remove an e(also, metals want to lose e-, but nonmetals do not)

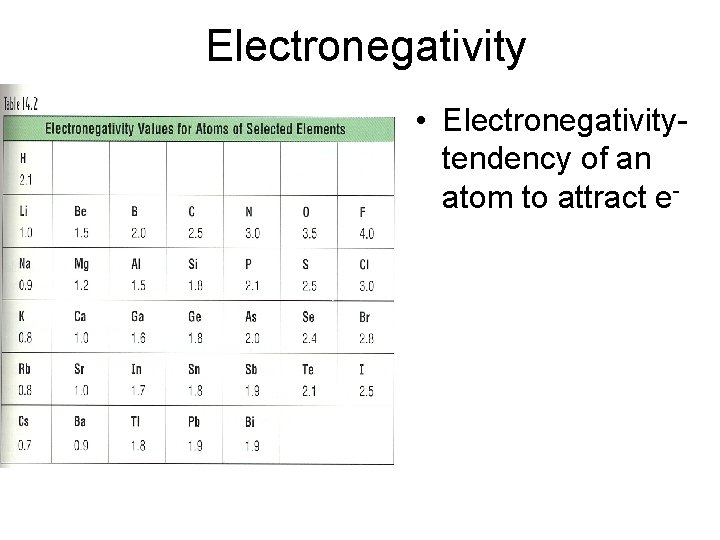
Electronegativity • Electronegativitytendency of an atom to attract e-

Electronegativity Trend • Group Trend – As you go down a column, electronegativity decreases As you go down, atomic size is increasing, so less attraction to its own e- and other atom’s e • Periodic Trend – As you go across a period (L to R), electronegativity increases As you go L to R, atomic size is decreasing, so there is more attraction to its own e- and other atom’s e-

Reactivity • Reactivity – tendency of an atom to react • Metals – lose e- when they react, so metals’ reactivity is based on lowest Ionization Energy (bottom/left corner) Low I. E = High Reactivity • Nonmetals – gain e- when they react, so nonmetals’ reactivity is based on high electronegativity (upper/right corner) High electronegativity = High reactivity

Metallic Character • Properties of a Metal – 1. Easy to shape 2. Conduct electricity 3. Shiny • • Group Trend – As you go down a column, metallic character increases Periodic Trend – As you go across a period (L to R), metallic character decreases (L to R, you are going from metals to non-metals

Region/Group Review What group or region of the Periodic Table are the following elements found in? 1. Strontium (Sr, #38) 2. Tantalum (Ta, #73) 3. Curium (Cm, #96) 4. Antimony (Sb, #51) 5. Sulfur (S, #16) 1. Alkaline Earth Metal, 2. Transition Metal, 3. Actinides (Transition Metal) 4. Metalloid, 5. Non-Metal

• 1. 2. 3. 4. 5. What physical appearance do they have? Strontium (Sr, #38) Tantalum (Ta, #73) Curium (Cm, #96) Antimony (Sb, #51) Sulfur (S, #16)

More Review Strontium (Sr, #38)

1. Tantalum (Ta, #73)

• Curium (Cm, #96)

• Antimony (Sb, #51)

• Sulfur (S, #16)

• What will they do with their electrons in a chemical reaction? 1. Strontium (Sr, #38) 2. Tantalum (Ta, #73) 3. Curium (Cm, #96) 4. Antimony (Sb, #51) 5. Sulfur (S, #16)

1. 2. 3. 4. 5. Strontium (Sr, #38)—lose two electrons Tantalum (Ta, #73)—unpredictable Curium (Cm, #96)—unpredictable Antimony (Sb, #51)—gain 3 electrons Sulfur (S, #16)—gain 2 electrons