Periodic Trends Elemental Properties and Patterns Periodic Trends












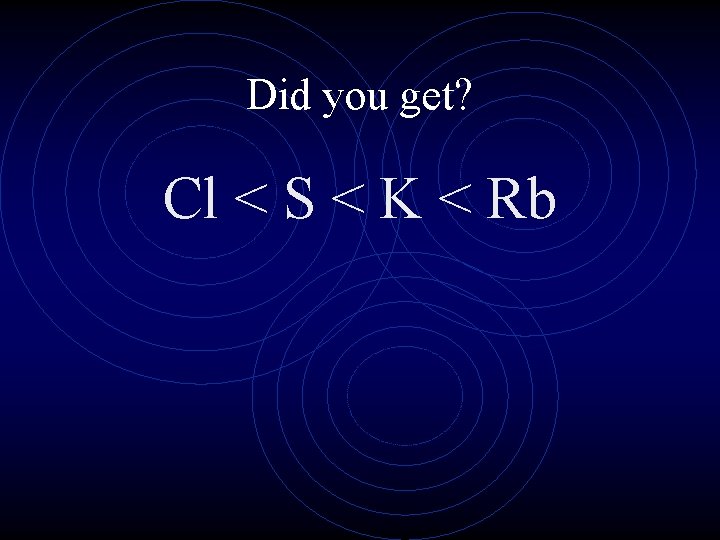











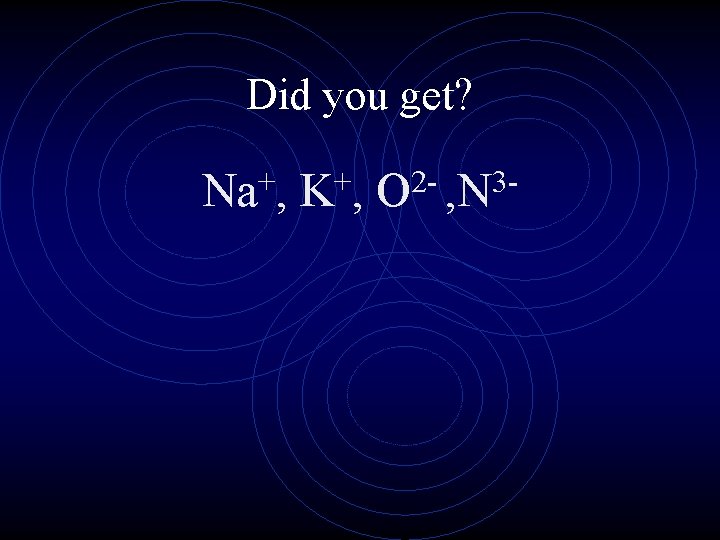









- Slides: 43

Periodic Trends Elemental Properties and Patterns

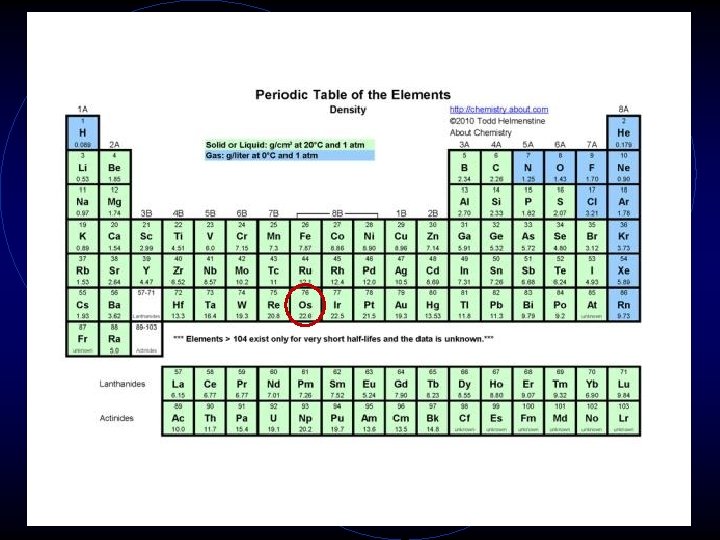
Periodic Trends • There are several properties that can be predicted by an elements position on the periodic table. • Example: Density ( • Density increases down the groups and towards the center of the periodic table (Osmium has the highest density, Hydrogen has the lowest density)

Density • Osmium (Z=76) has the highest density. • Why not Hassium (Z=108)? • Hassium only exists for a very short time so we have not been able to do density experiments to see what the true density of this element is. • So how do we figure out if an element is more dense? The closer it is to Osmium means it will be more dense.


Effective Nuclear Charge (Zeff) • The effective nuclear charge (Zeff ) of an atom is basically how well it is able to hold on to its most loosely held electron. • A higher effective nuclear charge means it will take more energy to rip off that electron from an atom.

Atomic Radius • The effective nuclear charge will determine the size of the atom or the atomic radius. • If an atom has more effective nuclear charge, the nucleus is pulling the electrons closer to it, making the atomic radius smaller. • If there is less effective nuclear charge, the nucleus is not able to pull those electrons in as well, and the atomic radius will be larger

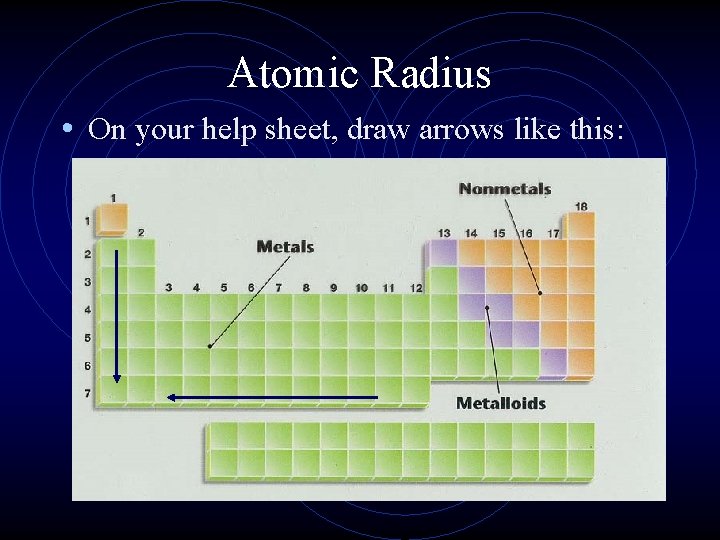
Atomic Radius • The trend across a horizontal period is as we move from left to right the atomic radius decreases. • Why?

Atomic Radius • The trend for atomic radius in a vertical column is to go from smaller at the top to larger at the bottom of the family. • Why?

Effective Nuclear Charge • The effective nuclear charge of an atom is primarily determined by: 1. The nuclear charge -The number of protons 2. The shielding effect -the number of energy levels

The Nuclear Charge • The size of atoms in the same period (row) is determined by the nuclear charge (the number of protons in the nucleus) • As we move across a period, we increase the amount of protons in the nucleus, meaning more positively charged particles to attract the negative electrons (more nuclear charge)

The Shielding Effect • The shielding effect is energy levels between the nucleus and the outmost electrons in an atom shield or lessen the hold of the nucleus on the outermost electrons • More energy levels means more of a shield preventing the outer electrons from being pulled in by the nucleus (less effective nuclear charge)

The Shielding Effect

Atomic Radius • On your help sheet, draw arrows like this:

Rank the atoms from smallest to largest K, S, Rb, Cl
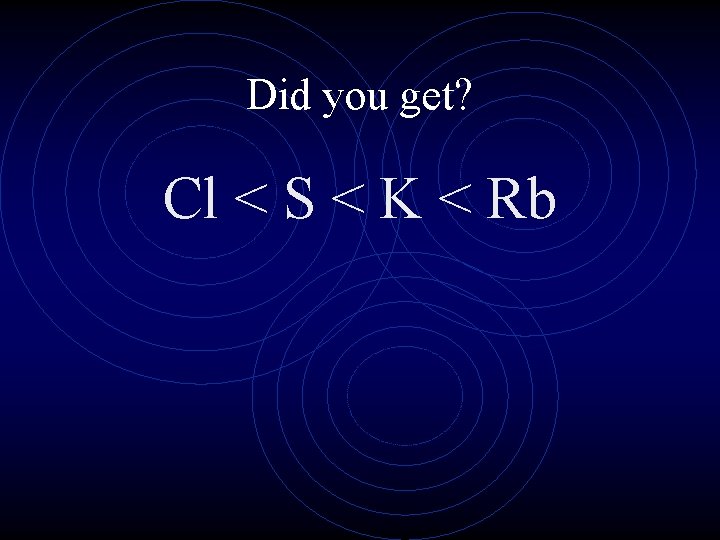
Did you get? Cl < S < K < Rb

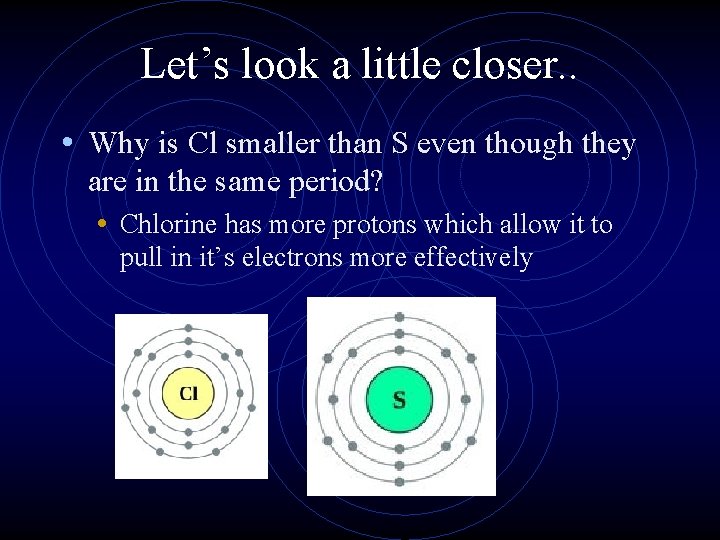
Let’s look a little closer. . • Why is Cl smaller than S even though they are in the same period? • Chlorine has more protons which allow it to pull in it’s electrons more effectively

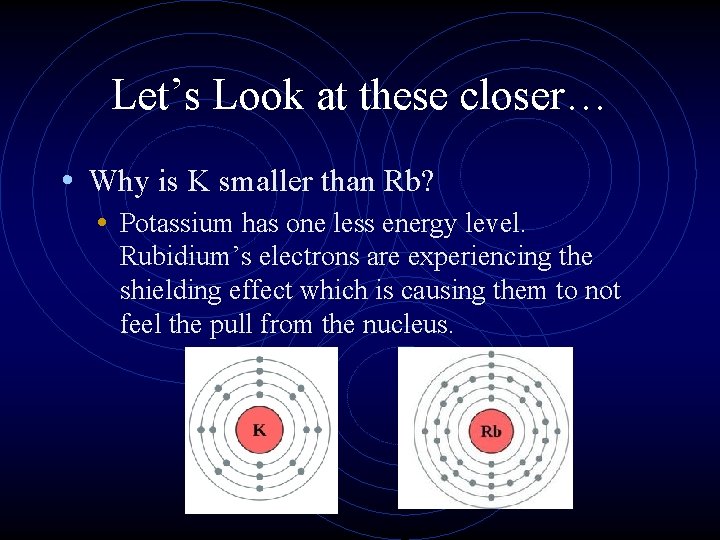
Let’s Look at these closer… • Why is K smaller than Rb? • Potassium has one less energy level. Rubidium’s electrons are experiencing the shielding effect which is causing them to not feel the pull from the nucleus.

Which atom would you predict to be larger? Carbon or Nitrogen?

Why is a carbon atom larger than a nitrogen atom?

Why is a carbon atom larger than a nitrogen atom? • The 7 protons in a nitrogen atom are more effective at pulling in the two energy levels then the 6 protons in a carbon atom.

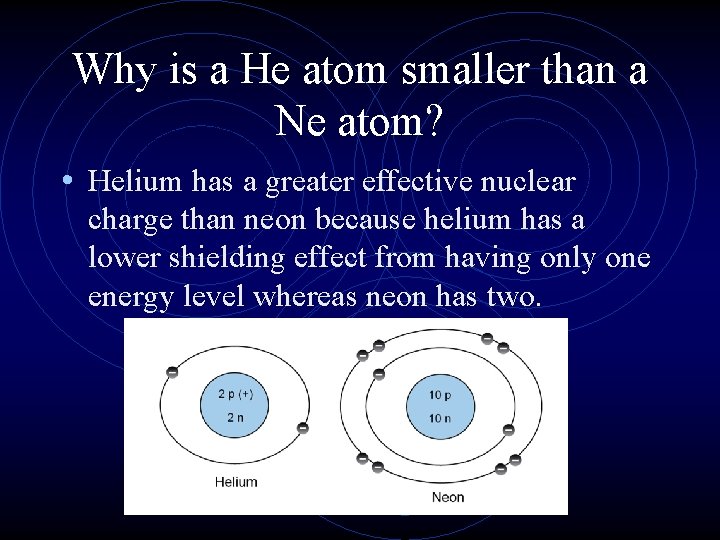
Why is a He atom smaller than a Ne atom?

Why is a He atom smaller than a Ne atom? • Helium has a greater effective nuclear charge than neon because helium has a lower shielding effect from having only one energy level whereas neon has two.

Chemical Reactivity • Metals tend to lose electrons when reacting • Large metal atoms are more reactive. • Nonmetals tend to gain electrons when reacting. • Small nonmetal atoms are more reactive.

Chemical Reactivity • Metals increase in reactivity left and down • Nonmetals become more reactive up and to the right. • Most reactive metal is? Francium • Most reactive nonmetals is? Fluorine

Francium • Francium is the least stable naturally occurring element with a half-life of only 22 minutes. • It has been calculate that there is at most 30 g of Francium in the Earth’s crust at any time.

Fluorine • The only elements it doesn't vigorously react with are oxygen, helium, neon, and argon. • It is one of the few elements that will form compounds with noble gases xenon, krypton, and radon.

Overall Reactivity • Your help sheet will look like this: 0

Ionic Radius • Ionic radius is the size of an ion. • Rules for Ionic Radius: • Anions are “always” larger than cations. • Ionic radius goes by the same rules as atomic radius (ions get larger as we move down and to the left) • We have to treat anions and cations separately (anions are bigger)

Rank the ions from smallest to largest + K, 3 N , + Na , 2 O
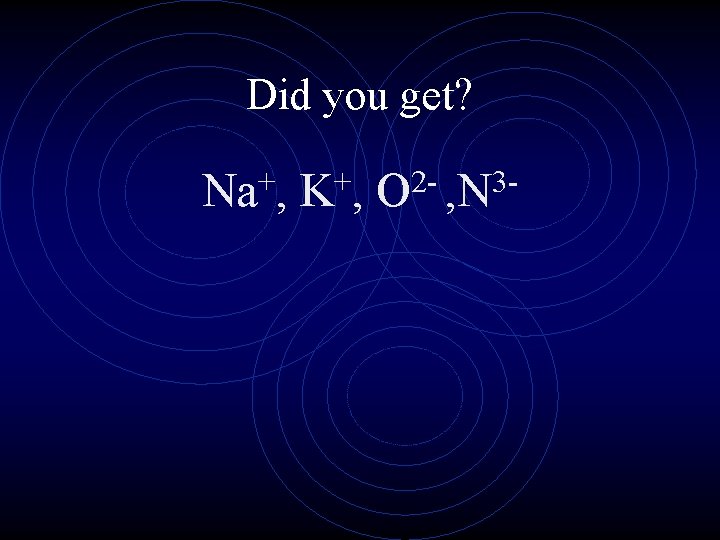
Did you get? + Na , + K, 23 O , N

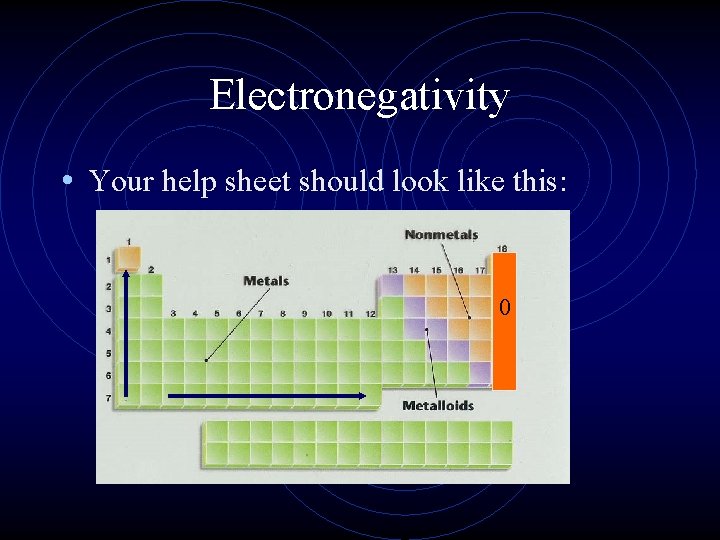
Electronegativity • Electronegativity is how likely an atom will be to gain electrons • The most electronegative element is fluorine. Why? • Fluorine is the smallest atom that needs one electron so it is the most likely atom to gain electrons

Electronegativity • The trend for electronegativity is that it increases when the atomic radius decreases. The smaller the atom, the more likely it will be able to add an electron.

Electronegativity • Your help sheet should look like this: 0

Ionization Energy • The energy needed to remove an electron from an atom is ionization energy.

First Ionization Energy • The First Ionization Energy is the energy needed to remove the first electron from an atom. • More energy is needed to remove electrons from a small atom (they feel a stronger pull from the nucleus), so the ionization energy has the same trend as electronegativity • As the radius gets smaller, more energy needed to remove an electron

Ionization Energy

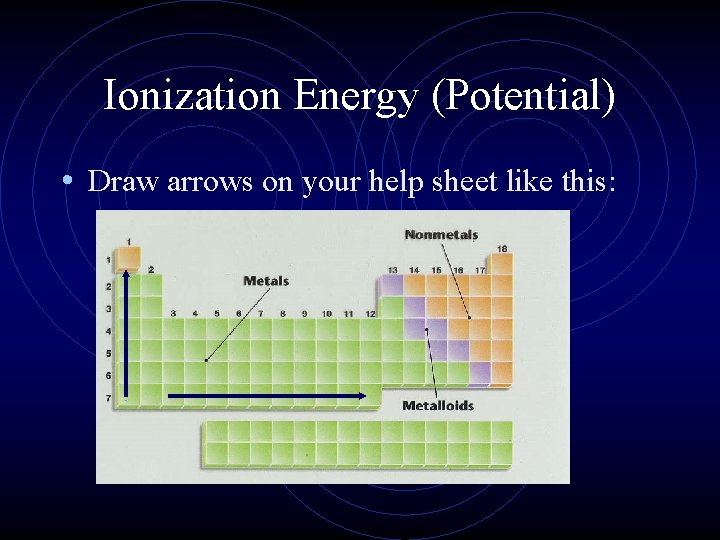
Ionization Energy (Potential) • Draw arrows on your help sheet like this:

First Ionization Energy • Francium has the lowest first ionization energy. Why?

First Ionization Energy • Francium has the lowest first ionization energy. Why? • Francium is the largest atom which means the electrons are easily taken off because they don’t feel as much of a pull from the nucleus (shielding effect)

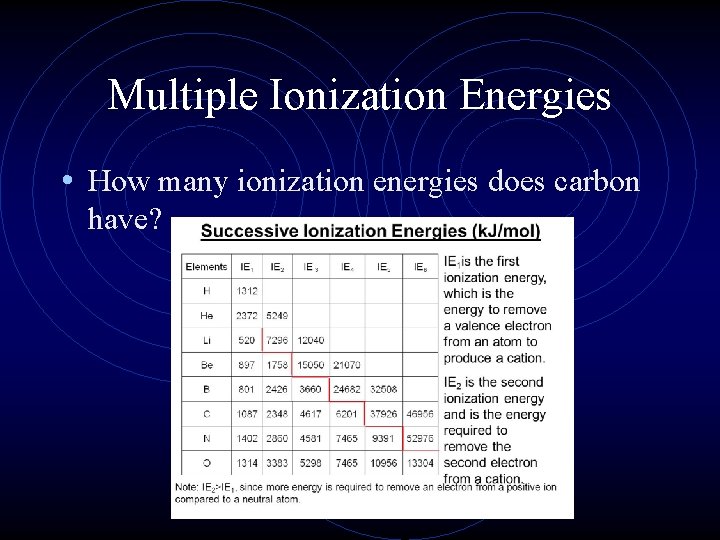
Multiple Ionization Energies • The first ionization energy is the energy needed to remove the first electron from an atom, so what about the second, third, etc. electrons? • Elements can have multiple ionization energies based on the number of electrons they have.

Multiple Ionization Energies • How many ionization energies does carbon have?

Multiple Ionization Energies • What happens to carbon’s size and effective nuclear charge as you remove electrons?

How would you relate ionization energy, atomic size, effective nuclear charge, reactivity, and electronegativity? • Larger atoms with low effective nuclear charge have low ionization energies and low electronegativity. This makes them very reactive because it is easier to remove electrons from them. • Smaller atoms with high effective nuclear charge have high ionization energies, and high electronegativities. This makes them very reactive because it is easy for them to pull electrons from other atoms.