Periodic Trends Atomic Radius Definition Half of the

- Slides: 16

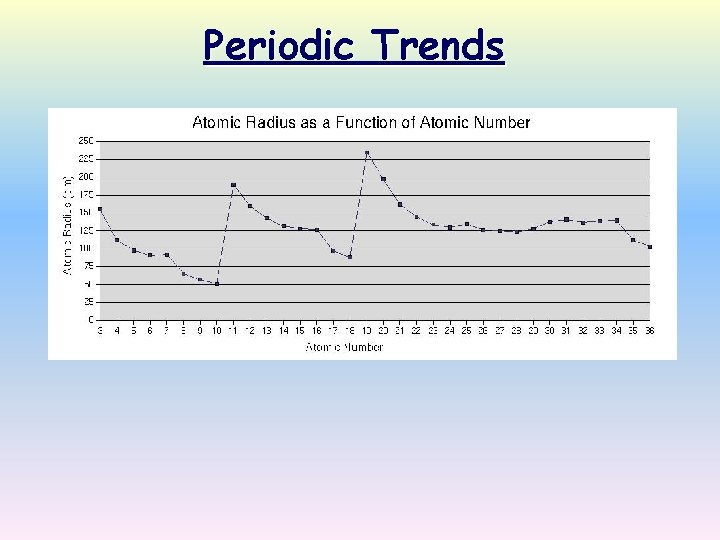
Periodic Trends

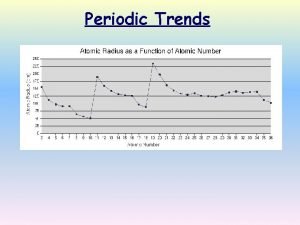
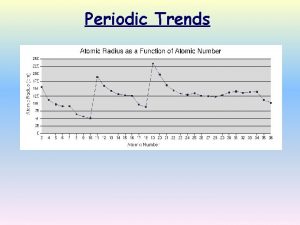
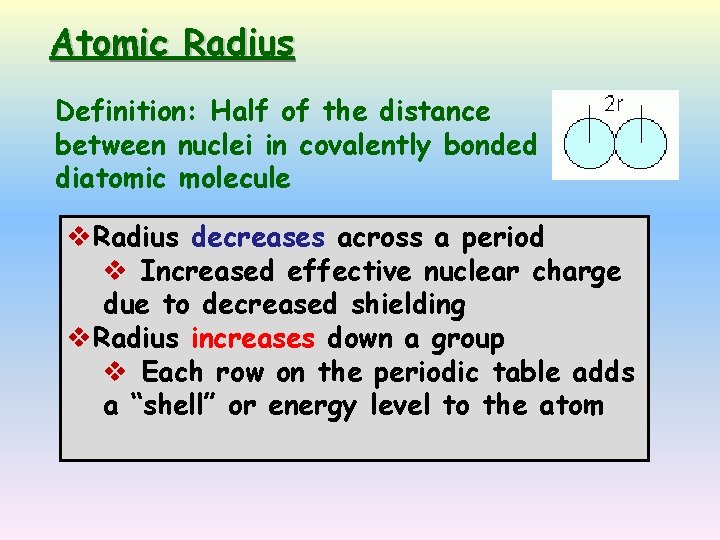
Atomic Radius Definition: Half of the distance between nuclei in covalently bonded diatomic molecule v. Radius decreases across a period v Increased effective nuclear charge due to decreased shielding v. Radius increases down a group v Each row on the periodic table adds a “shell” or energy level to the atom

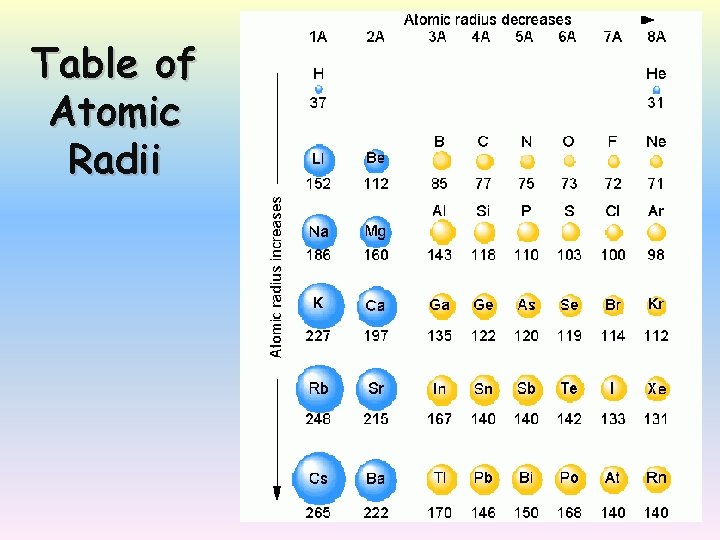
Table of Atomic Radii

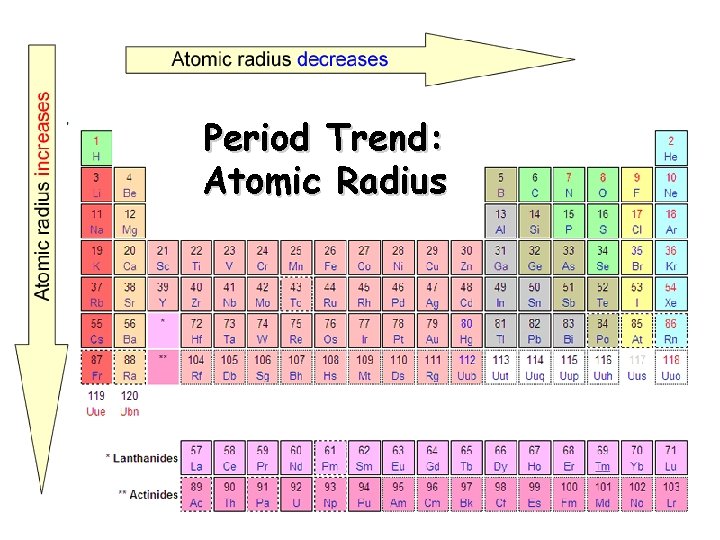
Period Trend: Atomic Radius

Ionization Energy Definition: the energy required to remove an electron from an atom q Increases for successive electrons taken from the same atom q. Tends to increase across a period q Electrons in the same quantum level do not shield as effectively as electrons in inner levels q Irregularities at half filled and filled sublevels due to extra repulsion of electrons paired in orbitals, making them easier to remove q Tends to decrease down a group q Outer electrons are farther from the nucleus and easier to remove

Ionization Energy: the energy required to remove an electron from an atom q Increases for successive electrons taken from the same atom q Tends to increase across a period Electrons in the same quantum level do not shield as effectively as electrons in inner levels Irregularities at half filled and filled sublevels due to extra repulsion of electrons paired in orbitals, making them easier to remove q Tends to decrease down a group Outer electrons are farther from the nucleus

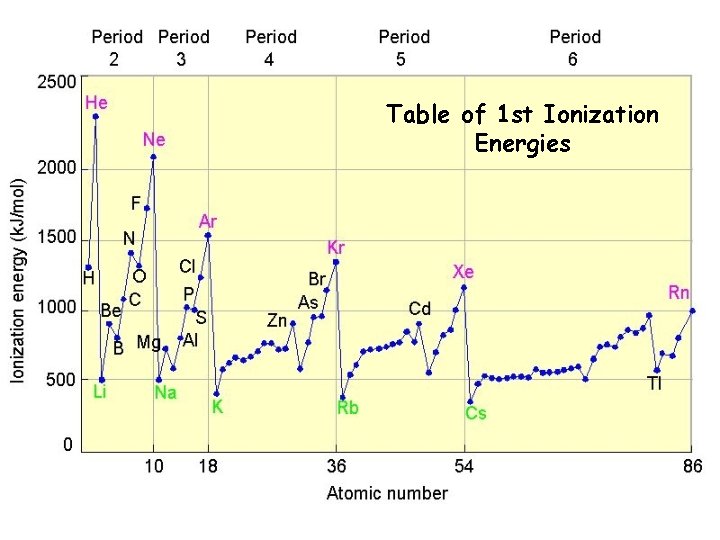
Table of 1 st Ionization Energies

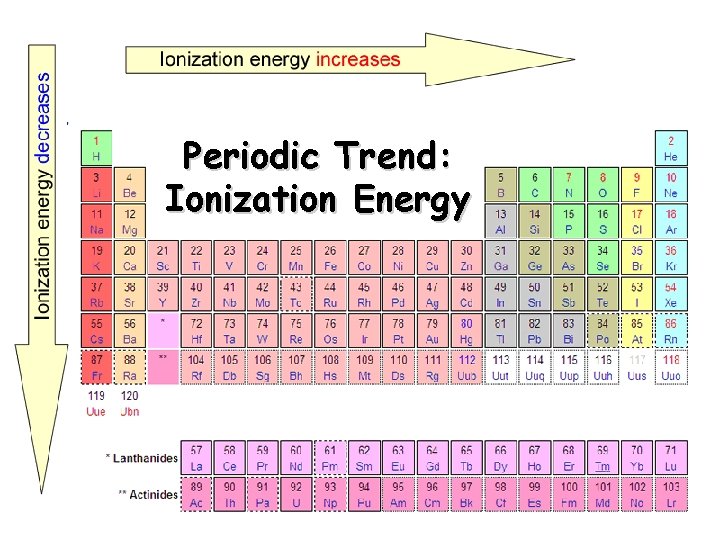
Periodic Trend: Ionization Energy

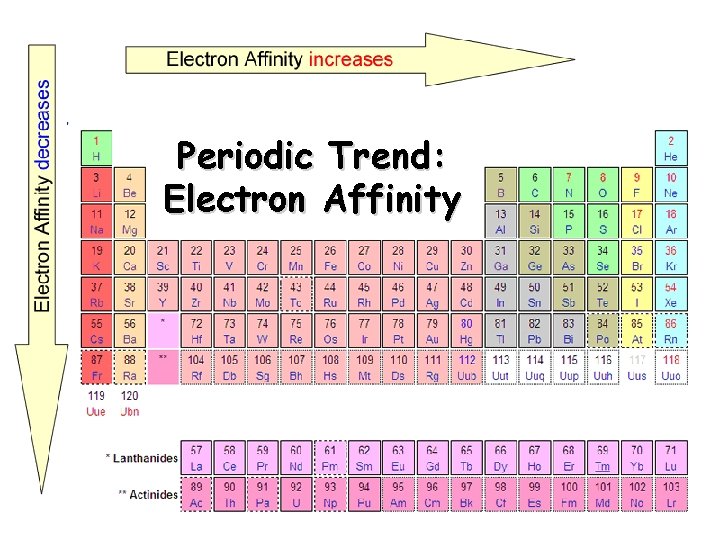
Electron Affinity Definition - the energy change associated with the addition of an electron q Affinity tends to increase across a period q Affinity tends to decrease as you go down in a period Electrons farther from the nucleus experience less nuclear attraction Some irregularities due to repulsive forces in the relatively small p orbitals

Periodic Trend: Electron Affinity

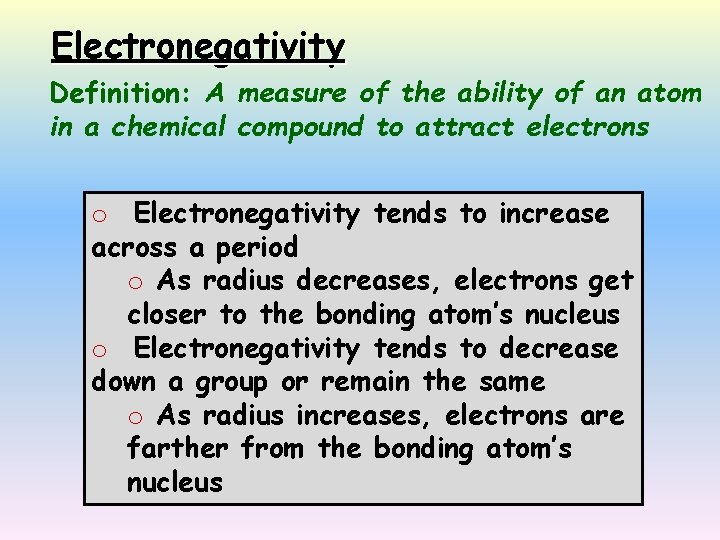
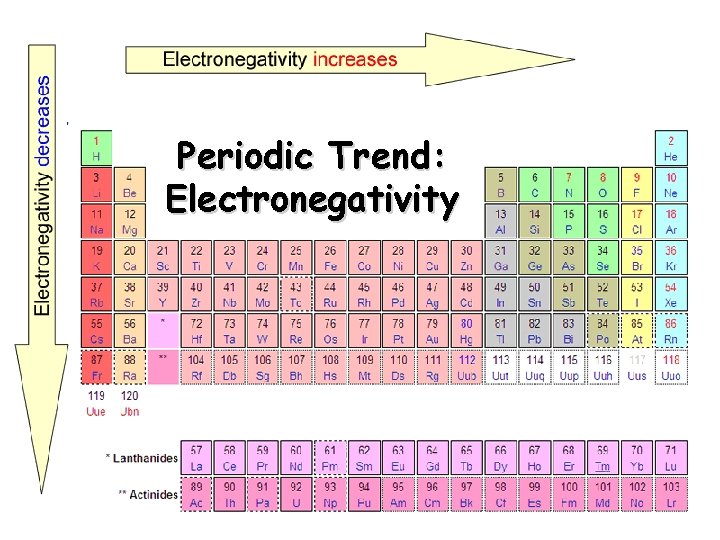
Electronegativity Definition: A measure of the ability of an atom in a chemical compound to attract electrons o Electronegativity tends to increase across a period o As radius decreases, electrons get closer to the bonding atom’s nucleus o Electronegativity tends to decrease down a group or remain the same o As radius increases, electrons are farther from the bonding atom’s nucleus

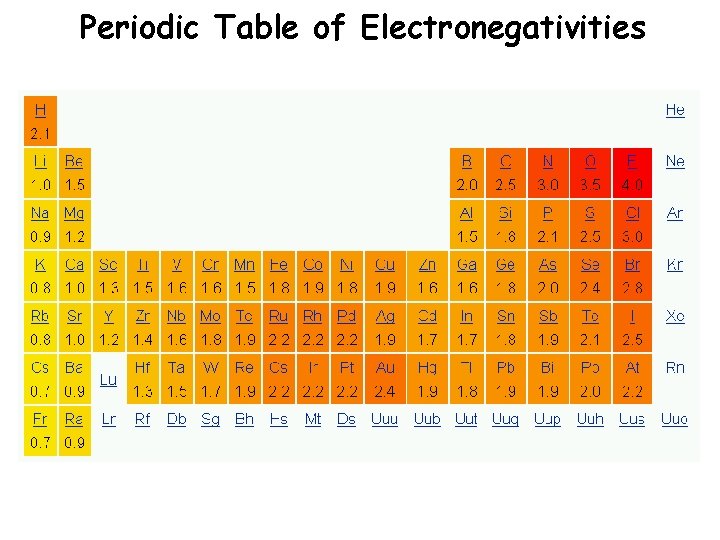
Periodic Table of Electronegativities

Periodic Trend: Electronegativity

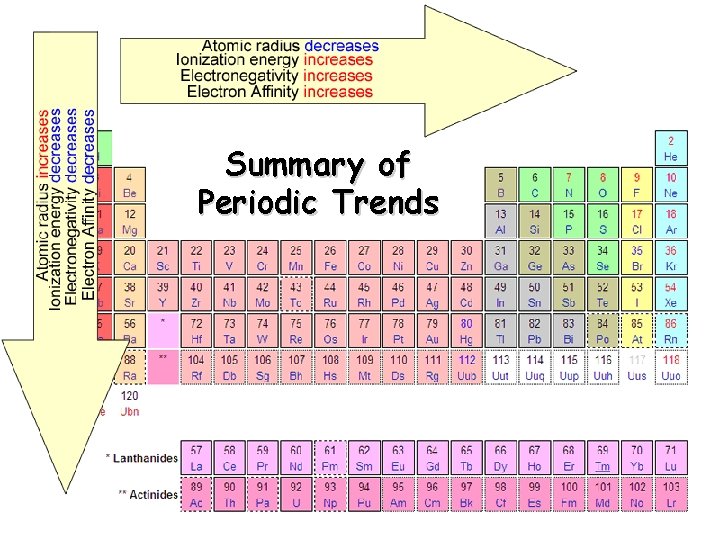
Summary of Periodic Trends

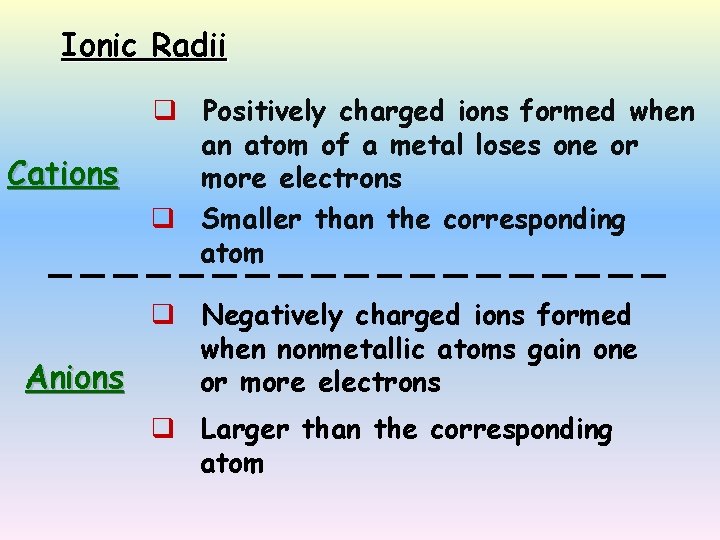
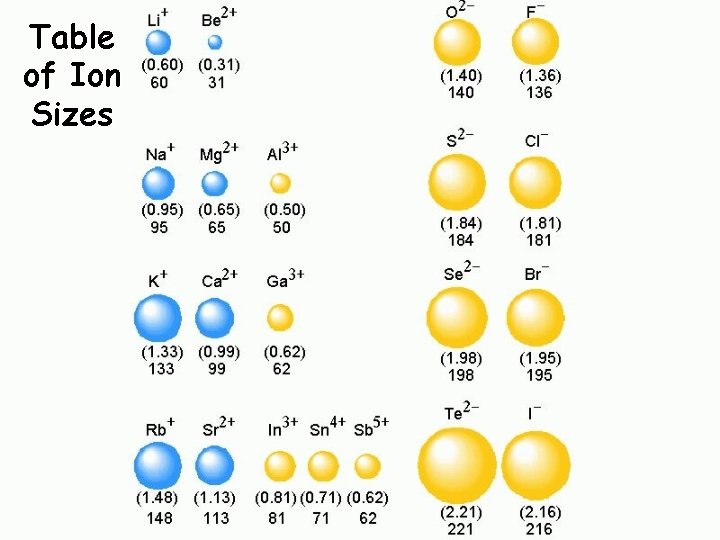
Ionic Radii Cations q Positively charged ions formed when an atom of a metal loses one or more electrons q Smaller than the corresponding atom q Negatively charged ions formed when nonmetallic atoms gain one Anions or more electrons q Larger than the corresponding atom

Table of Ion Sizes
 Small atomic radius
Small atomic radius 12 electrons
12 electrons Ionization energy trend periodic table
Ionization energy trend periodic table Periodic trebds
Periodic trebds Atomic number vs atomic radius
Atomic number vs atomic radius Atomic radius electronegativity
Atomic radius electronegativity Elements in period 2
Elements in period 2 Periodic trend definition
Periodic trend definition Ionic radius trend
Ionic radius trend Definition of atomic radius
Definition of atomic radius Ionic radius periodic table trend
Ionic radius periodic table trend Smallest atomic radius
Smallest atomic radius Smallest atomic radius
Smallest atomic radius Atomic radius increases when
Atomic radius increases when Largest atomic radius
Largest atomic radius Group 16 period 6
Group 16 period 6 Bromine sodium iodide
Bromine sodium iodide