Periodic Trends Atomic radius Ionic radius Electronegativity Ionization





















- Slides: 29

Periodic Trends Atomic radius Ionic radius Electronegativity Ionization energy

Your tool box • • • # protons Effective nuclear charge # electrons # of energy levels electron repulsion Core electrons/electron shielding F (coulomb’s law) q (coulomb’s law) r (coulomb’s law) – usually deals with # of energy levels

Coulomb’s Law • Relevant because we’re dealing with charged particles • r is the distance between the positive nucleus and a valence electron • q 1 is the charge of the nucleus (ENC) • q 2 is the charge of a single valence electron

Atomic Radius • Increases down a column because…. • Decreases across a row because….

Ionization Energy depends on size • Explain trend for atomic radius • “More energy is required to remove an electron closer to the nucleus. ”

Electronegativity • Depends on size and charge of nucleus

Ionic Radius • Depends on protons and electrons

Explain why the radius of the chlorine atom is smaller than the radius of the chloride ion, Cl-. (Radii : Cl atom = 0. 99Å; Cl- ion = 1. 81 Å) • • • # protons Now write an Effective nuclear charge explanation using the # electrons tools selected # of energy levels electron repulsion Core electrons/electron shielding F (coulomb’s law) q (coulomb’s law) r (coulomb’s law) – usually deals with # of energy levels

Chlorine and chloride have the same number of protons (17), but they have different numbers of electrons (chlorine has 17 while chloride has 18). The increase in electrons causes more electron repulsion, increasing the radius of chloride.

Tips • Make sure to mention things that are in common and things that are different (example to follow) • Use tool box words whenever possible • Mention both substances and use specific facts (# of protons, electrons, energy levels, etc. )

Tool box words you think are important • Common • Different

Tool box words you think are important • Common – Same number of protons/ENC • Different – # of electrons – Electron repulsion

Explain the fact that Calcium’s first ionization energy is larger than potassium’s. • • • # protons Now write an Effective nuclear charge explanation using the # electrons tools selected # of energy levels electron repulsion Core electrons/electron shielding F (coulomb’s law) q (coulomb’s law) r (coulomb’s law) – usually deals with # of energy levels

• Potassium and calcium have the same number of core electrons, but calcium has one additional proton. The larger nucleus means an increased effective nuclear charge (K is +1 while Ca is +2). Since the large nuclear charge on calcium increases the force of attraction between the nucleus and the valence electron according to coulomb’s law, more energy is required to remove the electron from calcium.

Homework Answers 1. Rotate around the group sharing answers to the homework (#3 starts) 2. If there any you can’t come to consensus on, please let me know

Today, we’re writing and talking…. First, we’re writing…. We have 5 examples that you will write explanations for

Your tool box • • • # protons Effective nuclear charge # electrons # of energy levels electron repulsion Core electrons/electron shielding F (coulomb’s law) q (coulomb’s law) r (coulomb’s law) – usually deals with # of energy levels

Which is larger: nitride or oxide? Explain why. • • • # protons Now write an Effective nuclear charge explanation using the tools selected # electrons # of energy levels electron repulsion Core electrons/electron shielding F (coulomb’s law) q (coulomb’s law) r (coulomb’s law) – usually deals with # of energy levels

Nitride vs Oxide • Nitride and oxide are isoelectronic (they have the same number of electrons – core and valence); however, the larger nucleus on oxide (8 protons for oxide while only 7 protons for nitride) means a larger force of attraction between the valence electrons and the nucleus, reducing the atomic radius of oxide.

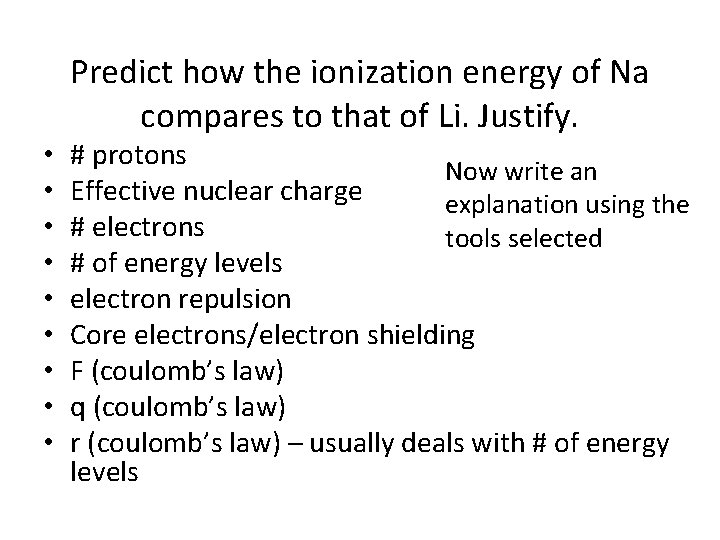
Predict how the ionization energy of Na compares to that of Li. Justify. • • • # protons Now write an Effective nuclear charge explanation using the # electrons tools selected # of energy levels electron repulsion Core electrons/electron shielding F (coulomb’s law) q (coulomb’s law) r (coulomb’s law) – usually deals with # of energy levels

Sodium’s valence electron is in the 3 rd energy level while Lithium’s valence electron is in the 2 nd energy level. The ionization energy of Na would be less than that of Li because the electron that would be removed is further from the nucleus. Also, the increased number of core electrons creates more electron shielding, making the valence electron easier to remove. Coulomb’s law indicates that electrons further from the nucleus have a lower attractive force holding them to the nucleus, so once again less energy is required to remove the electron from Na.

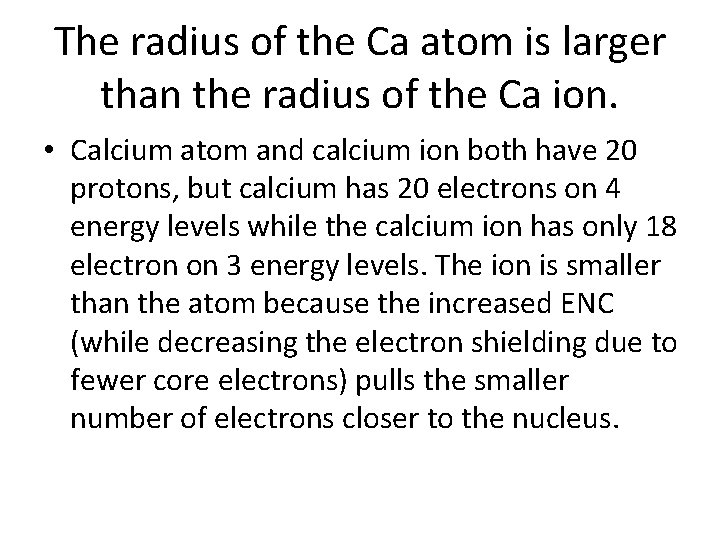
The radius of the Ca atom is larger than the radius of the Ca ion. Explain. • • • # protons Now write an Effective nuclear charge explanation using the # electrons tools selected # of energy levels electron repulsion Core electrons/electron shielding F (coulomb’s law) q (coulomb’s law) r (coulomb’s law) – usually deals with # of energy levels

The radius of the Ca atom is larger than the radius of the Ca ion. • Calcium atom and calcium ion both have 20 protons, but calcium has 20 electrons on 4 energy levels while the calcium ion has only 18 electron on 3 energy levels. The ion is smaller than the atom because the increased ENC (while decreasing the electron shielding due to fewer core electrons) pulls the smaller number of electrons closer to the nucleus.

Predict how the ionization energy of Na compares to that of Ne. Explain your reasoning.

Predict how the ionization energy of Na compares to that of Ne. The effective nuclear charge of Ne is much higher than Na (+8 compared to +1). Additionally, Na’s valence electron is found on the 3 rd energy level, while Ne’s is on the 2 nd energy level, meaning that neon is much smaller than sodium. Coulomb’s law says that electrons closer to the nucleus have a greater force of attraction, therefore more energy is required to remove the valence electron.

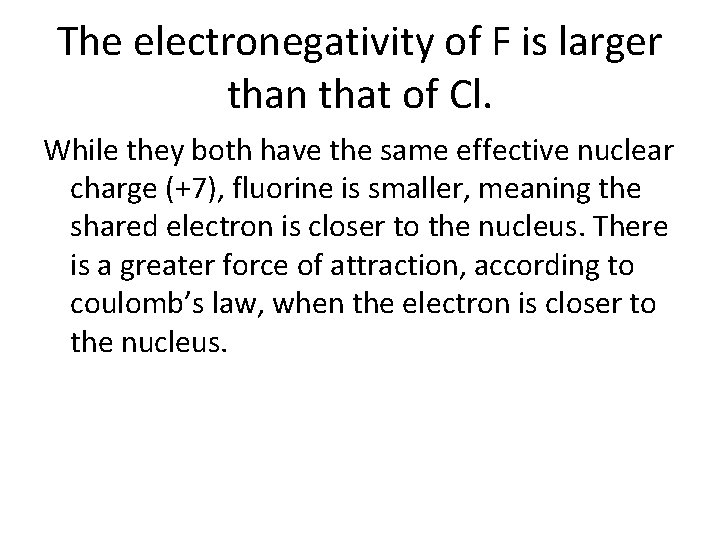
The electronegativity of F is larger than that of Cl. Explain. (For more information on electronegativity, click this link http: //www. chemguide. co. uk/atoms/bonding/electroneg. html) • # protons Now write an • Effective nuclear charge explanation using the • # electrons tools selected • # of energy levels • electron repulsion • Core electrons/electron shielding • F (coulomb’s law) • q (coulomb’s law) • r (coulomb’s law) – usually deals with # of energy levels

The electronegativity of F is larger than that of Cl. While they both have the same effective nuclear charge (+7), fluorine is smaller, meaning the shared electron is closer to the nucleus. There is a greater force of attraction, according to coulomb’s law, when the electron is closer to the nucleus.

Now, we’re talking Quiz your face partner using the blue cards

Practice Quiz • You will turn this quiz into me (but it will not count as a grade) • Write your name and class period on top of the lined side of the notecard; start on the lined side and answer this question: Which atom has a higher ionization energy, Al or Si? Explain why.