WHAT Radius Electronegativity Ionization Energy HOW WHY Periodic



- Slides: 11

WHAT Radius Electronegativity Ionization Energy HOW WHY

Periodic Trends • What is it? • How does it look on the Periodic Table? • Why does it happen?

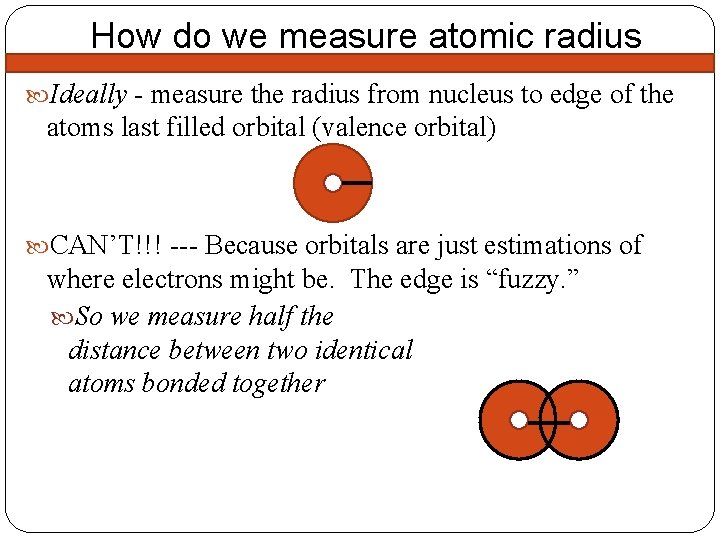
How do we measure atomic radius Ideally - measure the radius from nucleus to edge of the atoms last filled orbital (valence orbital) CAN’T!!! --- Because orbitals are just estimations of where electrons might be. The edge is “fuzzy. ” So we measure half the distance between two identical atoms bonded together

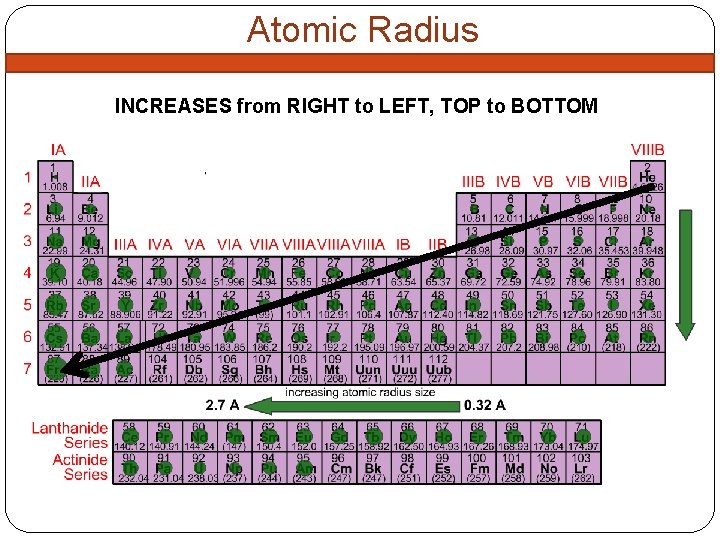
Atomic Radius INCREASES from RIGHT to LEFT, TOP to BOTTOM

Why does ATOMIC RADIUS increase the way it does? INCREASES DOWN a GROUP Adding more energy levels DECREASES LEFT to RIGHT ACROSS A PERIOD ADDING more protons, neutrons, electrons. SO WHY DOESN’T YOUR RADIUS GET BIGGER? ? More protons = the more they can PULL IN e= SMALLER radius GREATER EFFECTIVE NUCLEAR CHARGE = SMALLER RADIUS

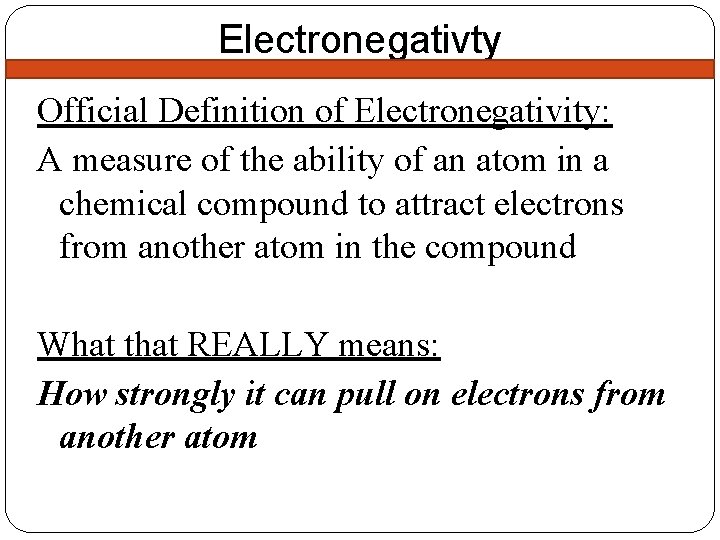
Electronegativty Official Definition of Electronegativity: A measure of the ability of an atom in a chemical compound to attract electrons from another atom in the compound What that REALLY means: How strongly it can pull on electrons from another atom

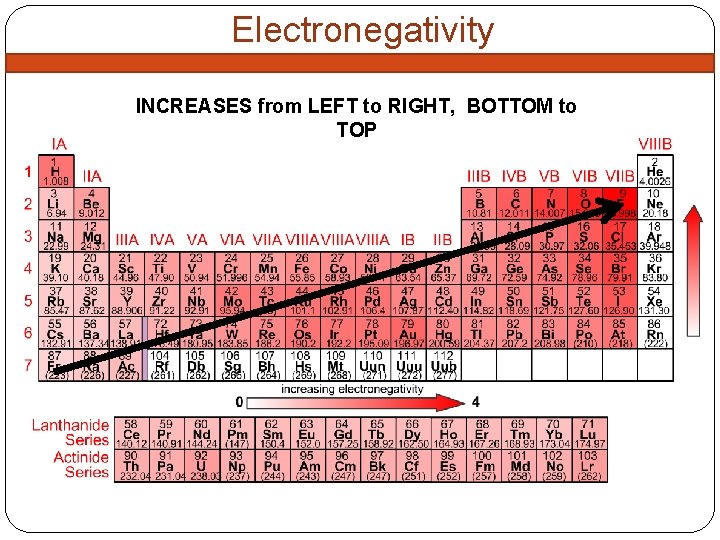
Electronegativity INCREASES from LEFT to RIGHT, BOTTOM to TOP

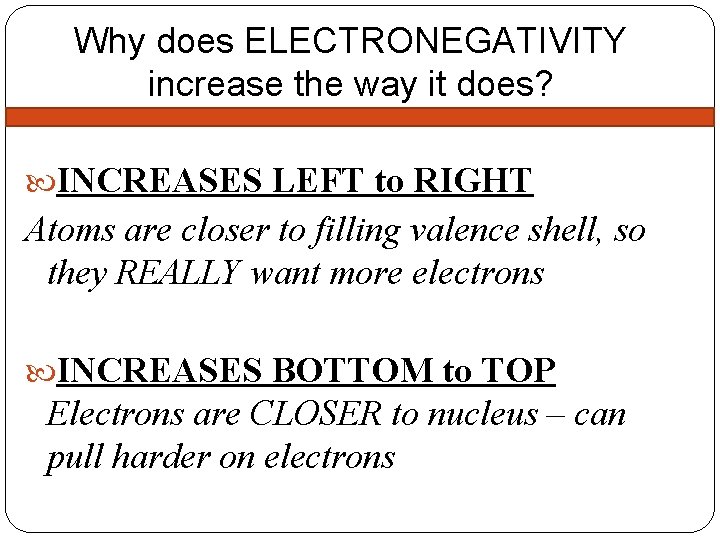
Why does ELECTRONEGATIVITY increase the way it does? INCREASES LEFT to RIGHT Atoms are closer to filling valence shell, so they REALLY want more electrons INCREASES BOTTOM to TOP Electrons are CLOSER to nucleus – can pull harder on electrons

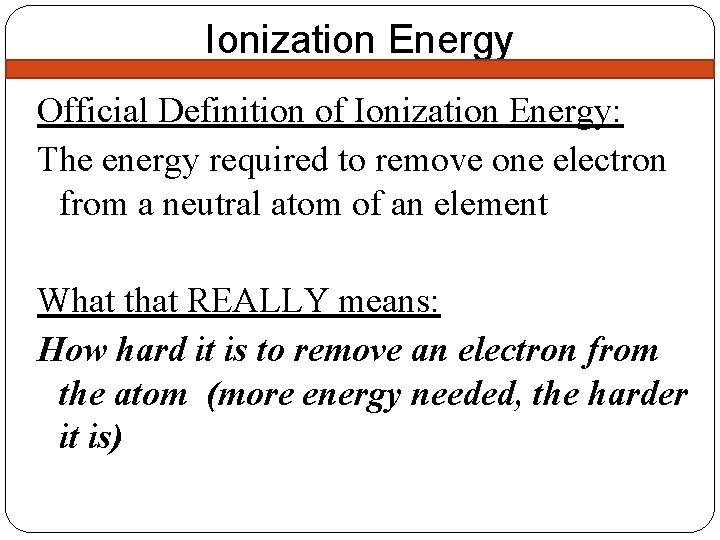
Ionization Energy Official Definition of Ionization Energy: The energy required to remove one electron from a neutral atom of an element What that REALLY means: How hard it is to remove an electron from the atom (more energy needed, the harder it is)

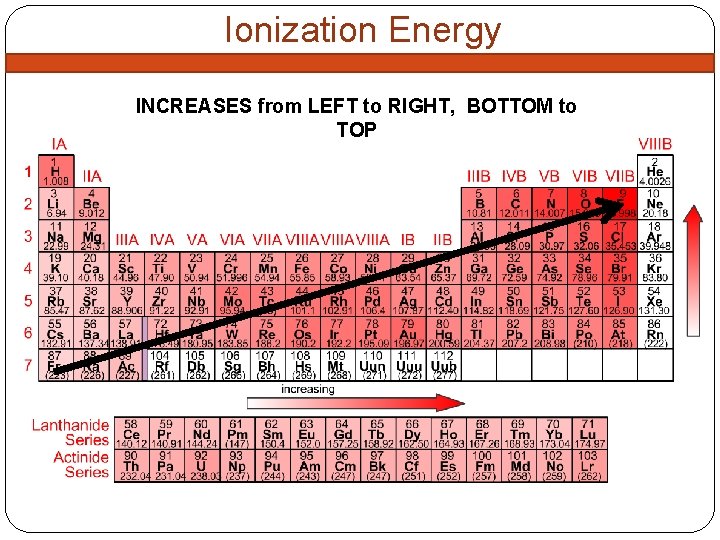
Ionization Energy INCREASES from LEFT to RIGHT, BOTTOM to TOP

Why does IONIZATION ENERGY increase the way it does? INCREASES LEFT to RIGHT atoms want a full valence shell…they DONT want to give away their electrons IF they are almost full! INCREASES BOTTOM to TOP electrons are CLOSER nucleus so they are being held onto tightly