Unit 7 Periodic Trends Trends in Reactivity Decreases


















- Slides: 39

Unit 7 Periodic Trends

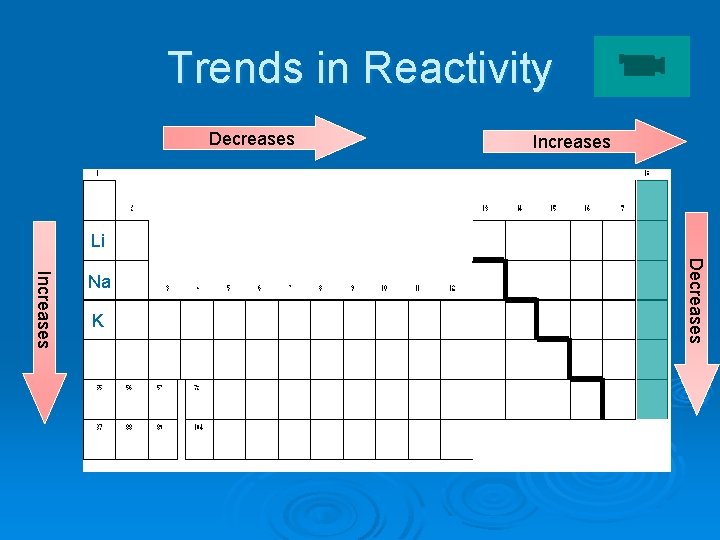
Trends in Reactivity Decreases Increases Li K Decreases Increases Na

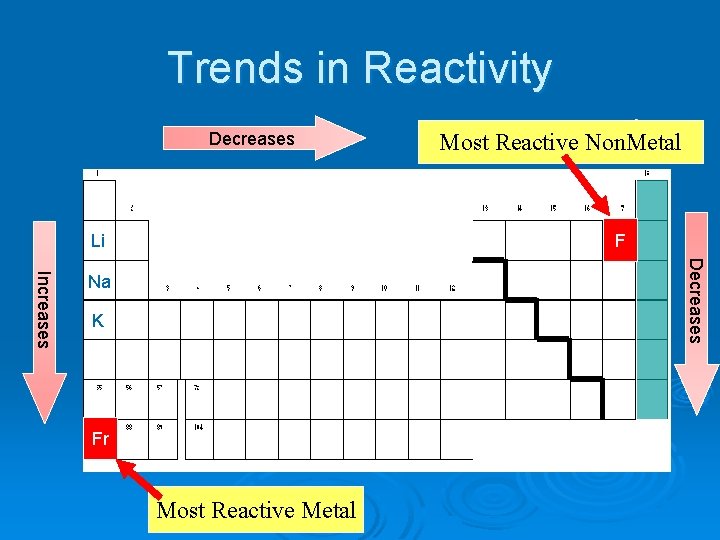
Trends in Reactivity Decreases Increases Most Reactive Non. Metal F Li Decreases Increases Na K Fr Most Reactive Metal

Which element is more reactive? Mg or Sr ? 2. K or Ca ? 3. Cl or I ? 4. Si or S ? 1.

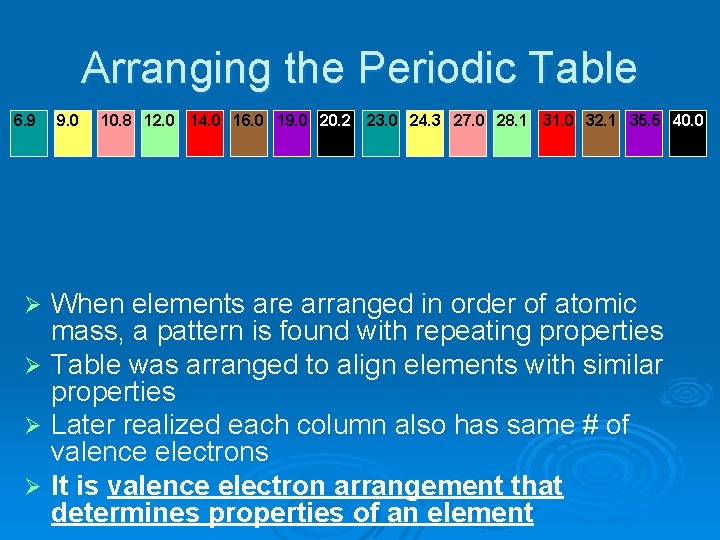
Arranging the Periodic Table 6. 9 9. 0 10. 8 12. 0 14. 0 16. 0 19. 0 20. 2 23. 0 24. 3 27. 0 28. 1 31. 0 32. 1 35. 5 40. 0 When elements are arranged in order of atomic mass, a pattern is found with repeating properties Ø Table was arranged to align elements with similar properties Ø Later realized each column also has same # of valence electrons Ø It is valence electron arrangement that determines properties of an element Ø

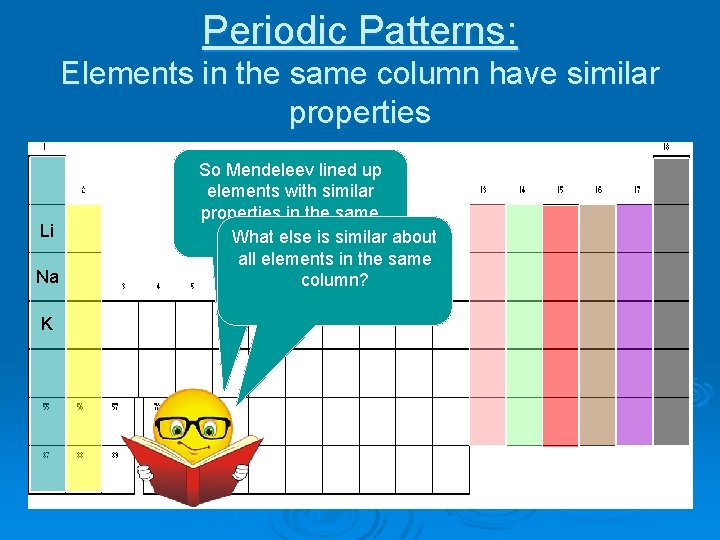
Periodic Patterns: Elements in the same column have similar properties Li Na K So Mendeleev lined up elements with similar properties in the same column. What else is similar about all elements in the same column?

Arranging the Periodic Table Ø Later realized each column also has same # of valence electrons Ø It is valence electron arrangement that determines properties of an element

Mendeleev Ø Mendeleev left blank spaces where no elements matched properties Ø Mendeleev predicted the existence of undiscovered elements Ø Mendeleev predicted the properties of these undiscovered elements

Atomic Attractions Ø What holds the earth in its orbit? Ø What holds the electrons in the atom?

Trends in Attractions Moving down a column Li Focus on attractions between nucleus and valence electrons Na K

Trends in Attractions Moving down a column Li Which element has a stronger hold on its valence electron? Na K

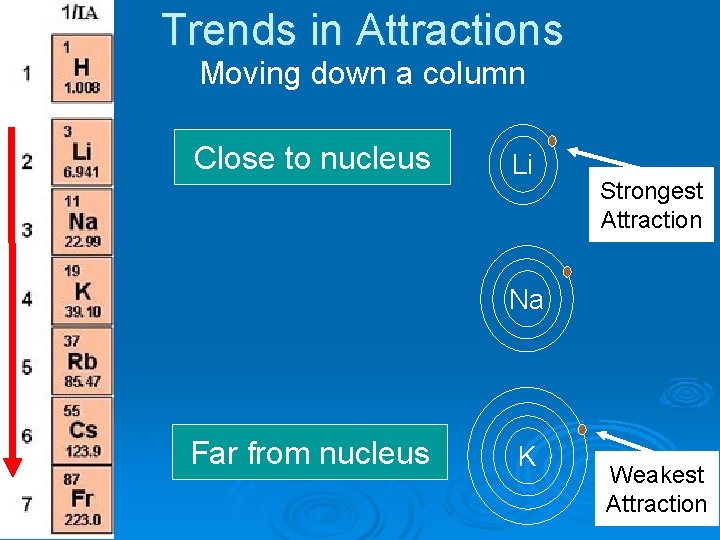
Trends in Attractions Moving down a column Close to nucleus Li Strongest Attraction Na Far from nucleus K Weakest Attraction

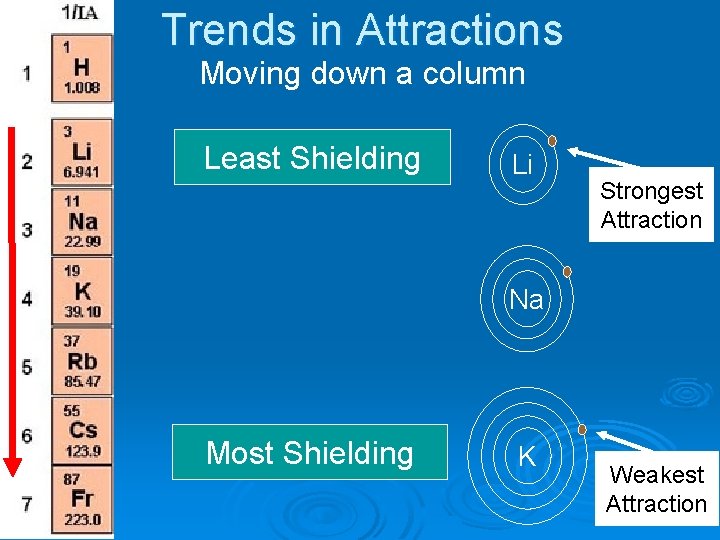
Trends in Attractions Moving down a column Least Shielding Li Strongest Attraction Na Most Shielding K Weakest Attraction

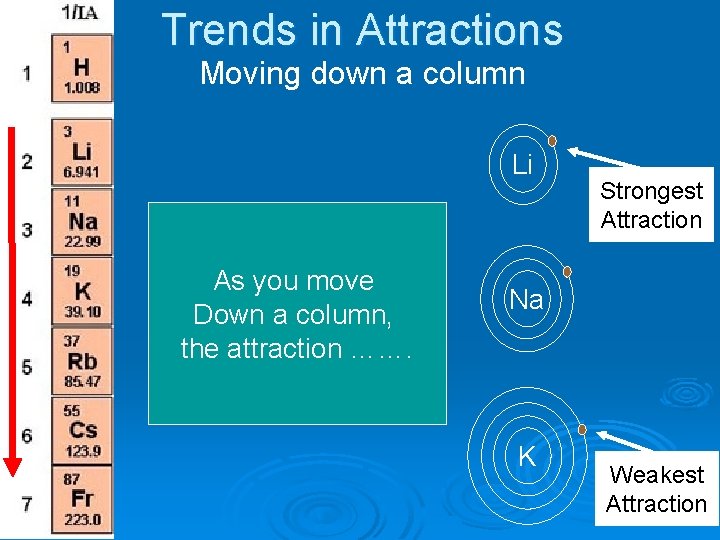
Trends in Attractions Moving down a column Li As you move Down a column, the attraction ……. Strongest Attraction Na K Weakest Attraction

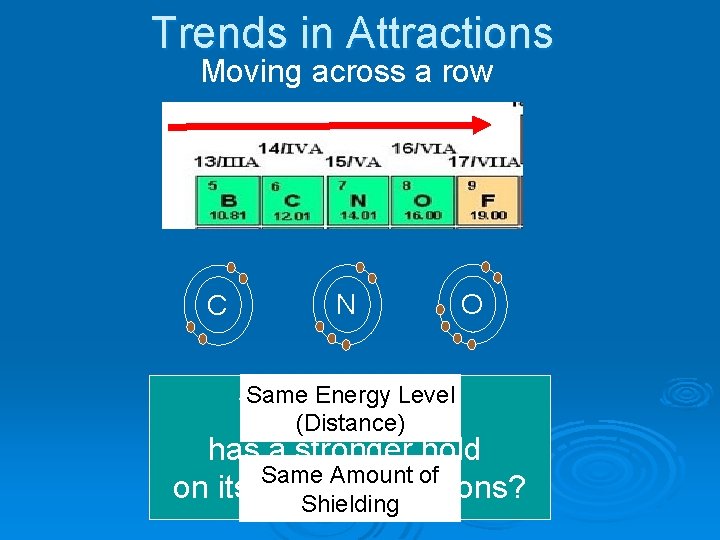
Trends in Attractions Moving across a row C N O

Trends in Attractions Moving across a row C N Same Energy Level Which element (Distance) O has a stronger hold Same Amount of on its valence electrons? Shielding

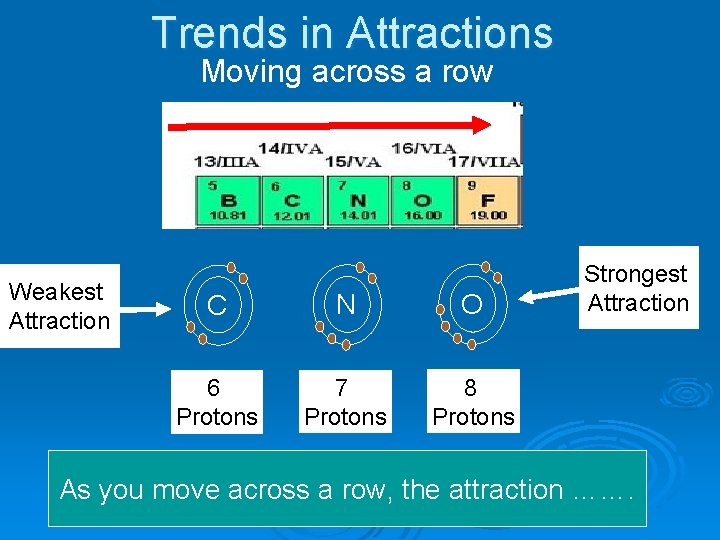
Trends in Attractions Moving across a row Weakest Attraction C N O 6 Protons 7 Protons 8 Protons Strongest Attraction As you move across a row, the attraction …….

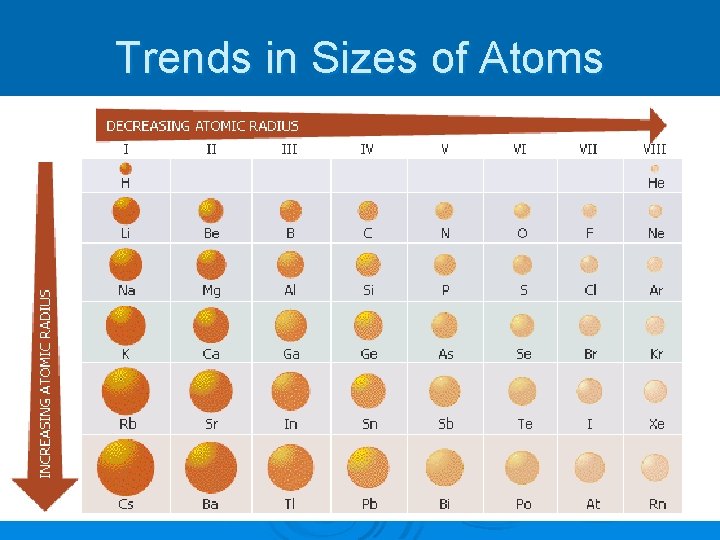
Which atom is bigger? ØF or Cl ? Li Na Going down any column, the atoms get bigger because you are adding energy levels K

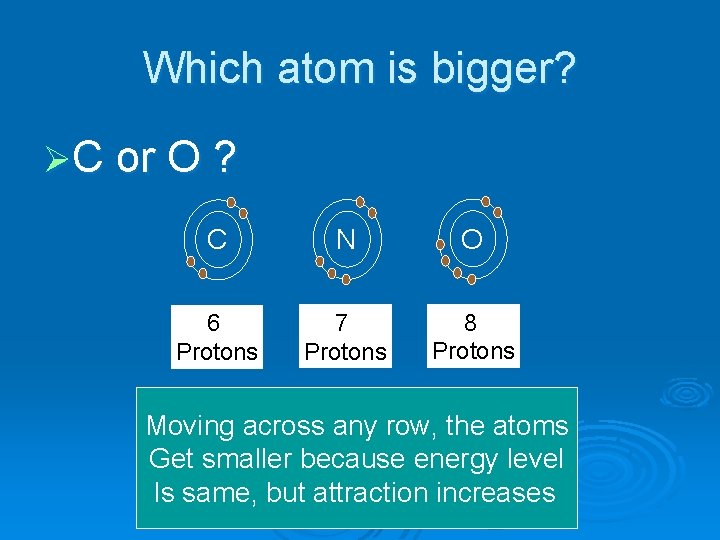
Which atom is bigger? ØC or O ? C N O 6 Protons 7 Protons 8 Protons Moving across any row, the atoms Get smaller because energy level Is same, but attraction increases

Trends in Sizes of Atoms

Which element is bigger? Mg or Sr ? 2. K or Ca ? 3. Cl or I ? 4. Si or S ? 1.

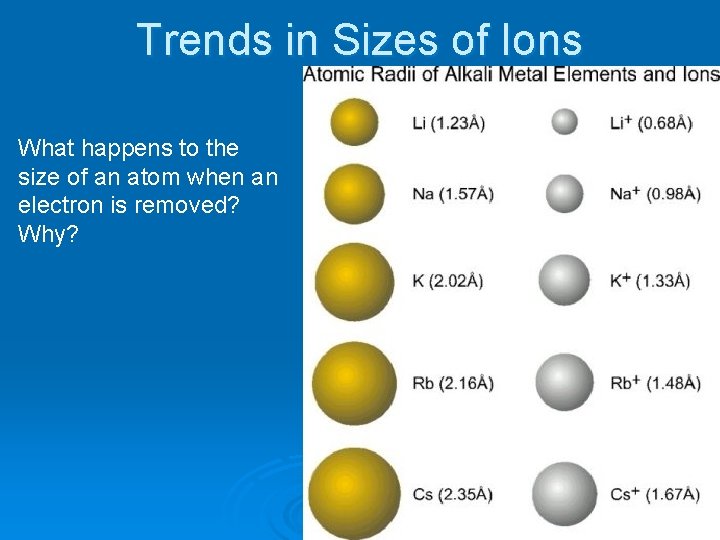
Trends in Sizes of Ions What happens to the size of an atom when an electron is removed? Why?

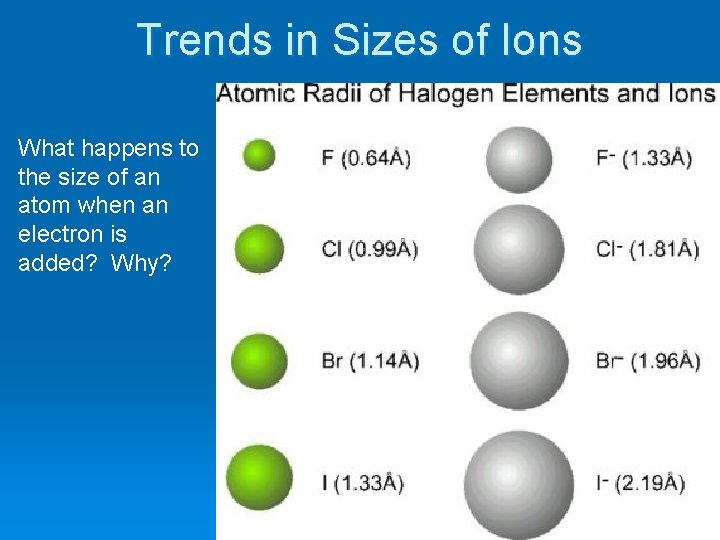
Trends in Sizes of Ions What happens to the size of an atom when an electron is added? Why?

Trends in Sizes of Ions of Different Elements Ø Who is bigger K or Cl ? Ø Compare the two ions Protons Electrons + K 19 18 Cl 17 18

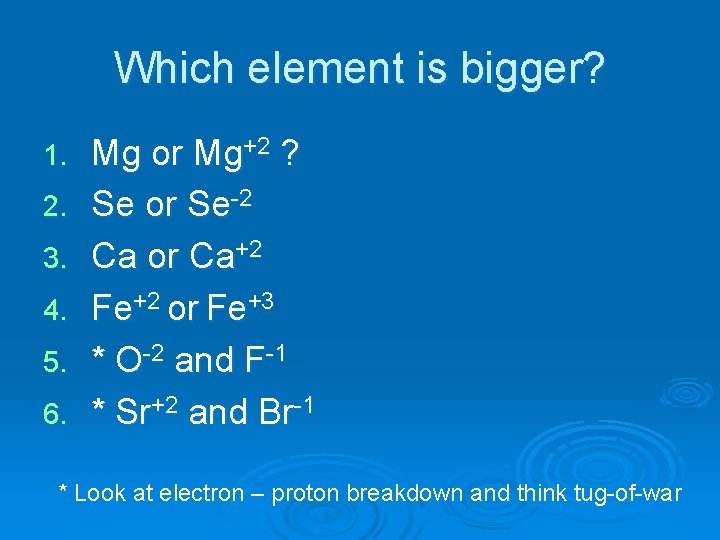
Which element is bigger? 1. 2. 3. 4. 5. 6. Mg or Mg+2 ? Se or Se-2 Ca or Ca+2 Fe+2 or Fe+3 * O-2 and F-1 * Sr+2 and Br-1 * Look at electron – proton breakdown and think tug-of-war

Ø Go to Ionization Energy Worksheet

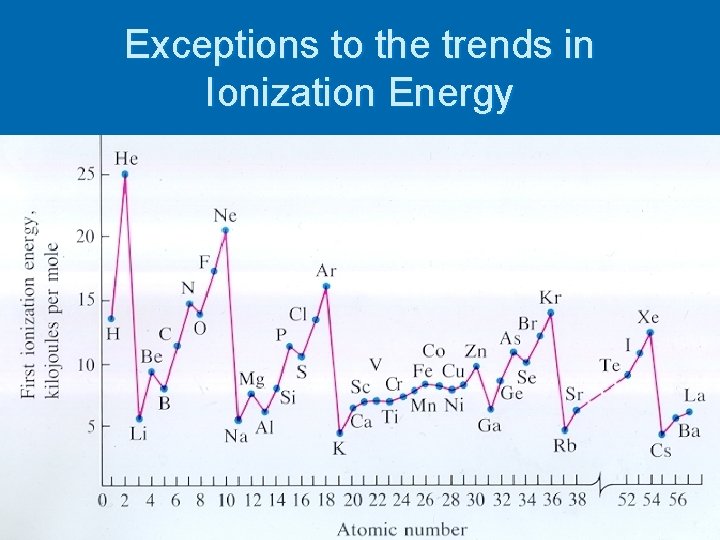
Exceptions to the trends in Ionization Energy

What Elements have the highest Ionization Energy ? Why?

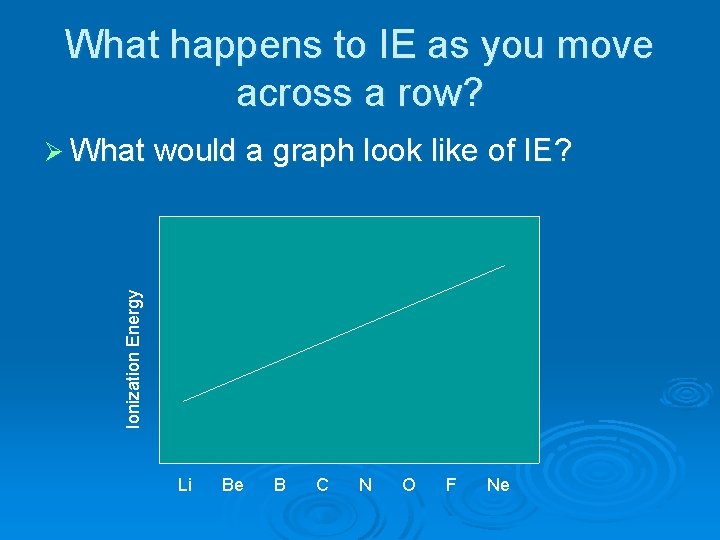
What happens to IE as you move across a row? Ionization Energy Ø What would a graph look like of IE? Li Be B C N O F Ne

Does the graph for row 2 appear as the trends predict?

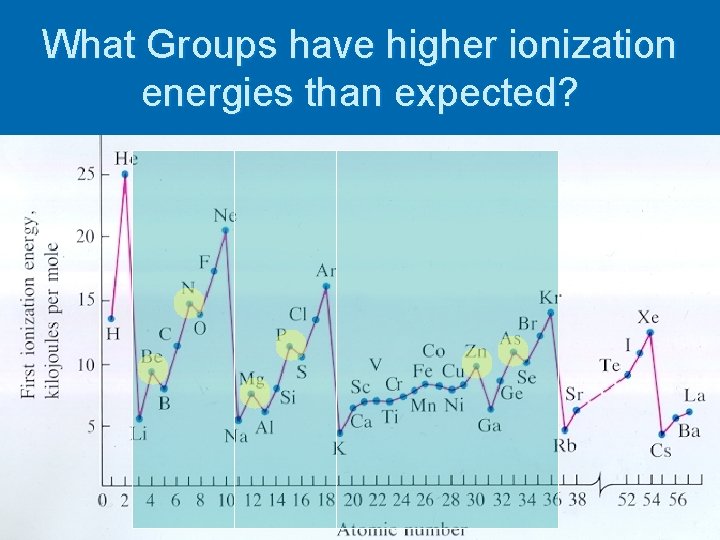
What Groups have higher ionization energies than expected?

Draw Orbital Notation for: Ø Group II: Mg Ø Group V: N Ø d 10 Group: Zn Why do you think these groups have higher ionization energies than expected?

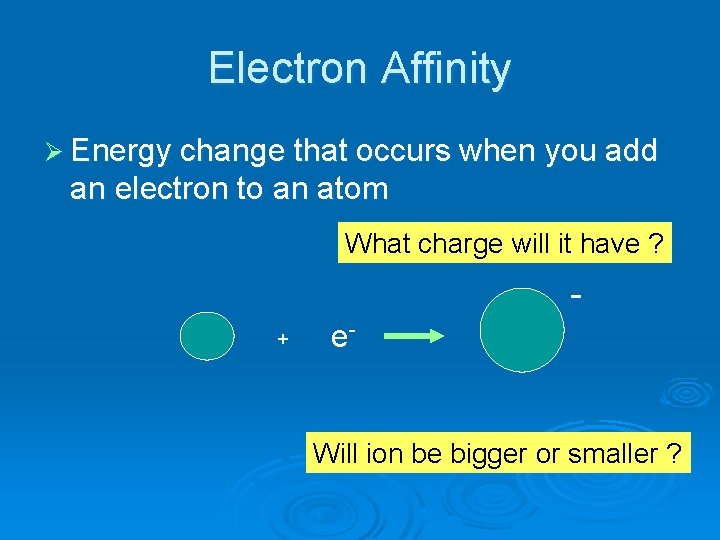
Electron Affinity Ø Energy change that occurs when you add an electron to an atom What charge will it have ? + e- Will ion be bigger or smaller ?

Trends in Electron Affinity What Groups would want e-’s? Ø What Groups would not want e-’s? Ø Turn to page 182 in text Ø What do you first notice about affinities? Ø

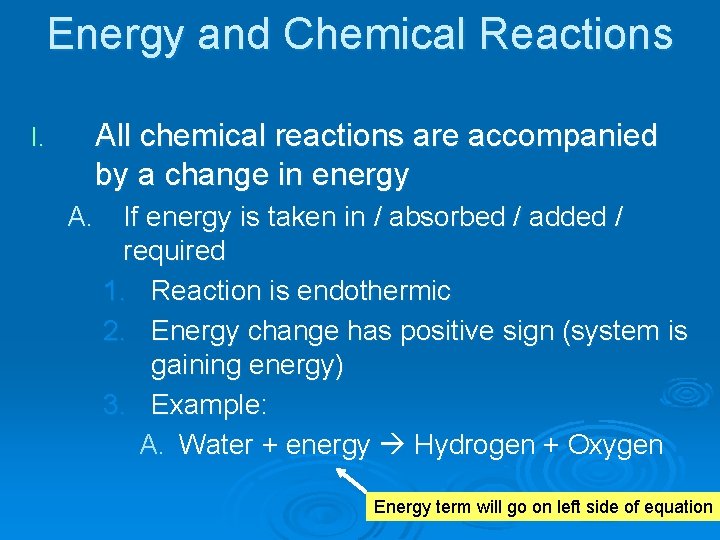
Energy and Chemical Reactions I. All chemical reactions are accompanied by a change in energy A. If energy is taken in / absorbed / added / required 1. Reaction is endothermic 2. Energy change has positive sign (system is gaining energy) 3. Example: A. Water + energy Hydrogen + Oxygen Energy term will go on left side of equation

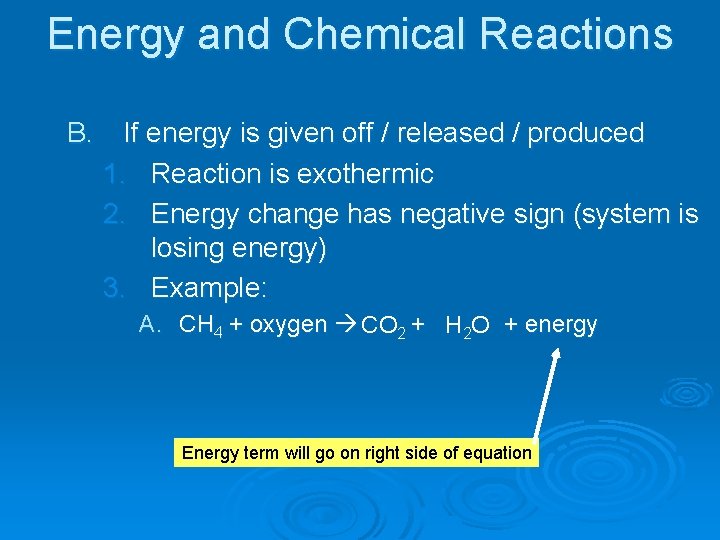
Energy and Chemical Reactions B. If energy is given off / released / produced 1. Reaction is exothermic 2. Energy change has negative sign (system is losing energy) 3. Example: A. CH 4 + oxygen CO 2 + H 2 O + energy Energy term will go on right side of equation

You turn: Label each reaction as endo or exothermic 1. 2. 3. 4. 5. When water changes from a liquid to a gas, it requires 44 k. J of energy. You body reacts glucose with oxygen to produce CO 2 and H 2 O. The energy change for this process is -2803 k. J. The melting of ice. KMn. O 4 + 42. 1 k. J K+ + Mn. O 4 The burning of coal.

Writing Equations with energy Show the removal of an e- from magnesium Show the addition of an e- to bromine

Review Practice Ca, Na, Hg, Al, Si, S, Cl+ Only include for #1 1. Arrange the above in order of increasing size 2. Arrange the above in order of increasing I. E. 3. Which would you predict to have a positive E. A? 4. Which would you expect to release the most energy when an electron is added to it?
 Slidetodoc
Slidetodoc Periodic trends in reactivity
Periodic trends in reactivity Periodic table with electronegativity and ionization energy
Periodic table with electronegativity and ionization energy Alien periodic table periodic trends answers
Alien periodic table periodic trends answers Reactivity of elements
Reactivity of elements Reactivity periodic trend
Reactivity periodic trend Is sodium a metal or nonmetal
Is sodium a metal or nonmetal Reactivity in periodic table
Reactivity in periodic table Properties of gas
Properties of gas Co = hr x sv
Co = hr x sv Stroke volume units
Stroke volume units Decreases monotonically
Decreases monotonically Why freezing point decreases on adding solute
Why freezing point decreases on adding solute Why freezing point decreases on adding solute
Why freezing point decreases on adding solute Which compounds solubility decreases most rapidly
Which compounds solubility decreases most rapidly Problem 3-11 describing business transactions
Problem 3-11 describing business transactions Li be b c n o
Li be b c n o Atomic size
Atomic size Trends in the periodic table labster
Trends in the periodic table labster Periodic trends cheat sheet
Periodic trends cheat sheet Oxygen periodic trends
Oxygen periodic trends Graphing periodic trends
Graphing periodic trends Oxidation trends periodic table
Oxidation trends periodic table Periodic trends electronegativity
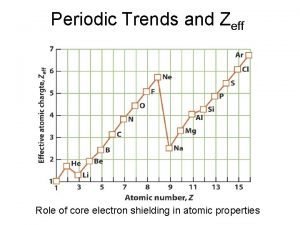
Periodic trends electronegativity Zeff trend
Zeff trend Periodic trends activity worksheet
Periodic trends activity worksheet Periodic trends practice questions
Periodic trends practice questions Summary of periodic trends
Summary of periodic trends Electron practice problems
Electron practice problems Coulomb's law periodic trends
Coulomb's law periodic trends Highest electron affinity
Highest electron affinity Increasing atomic size
Increasing atomic size Is sulfer a cation or anion
Is sulfer a cation or anion Periodic trends definition
Periodic trends definition Dp periodic table
Dp periodic table Periodic table trends
Periodic table trends Periodic table trends
Periodic table trends Nuclear charge definition
Nuclear charge definition Periodic table with properties
Periodic table with properties Ionization energy trend periodic table
Ionization energy trend periodic table