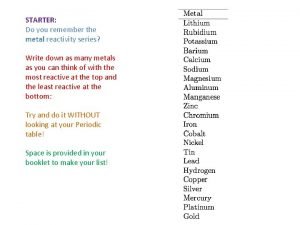
6 8 NOTES Metal Reactivity Trends Metal Reactivity














- Slides: 17

6. 8 – NOTES Metal Reactivity Trends

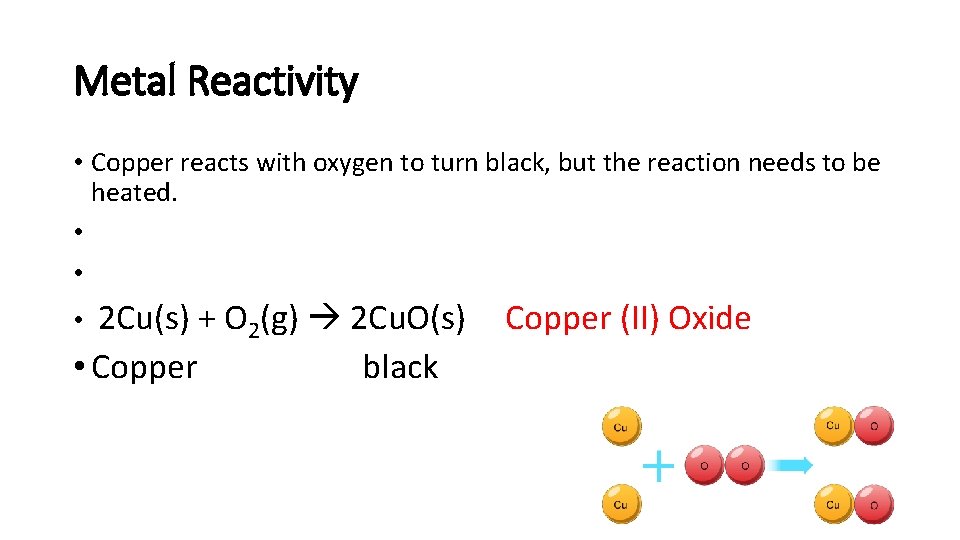
Metal Reactivity • Copper reacts with oxygen to turn black, but the reaction needs to be heated. • • 2 Cu(s) + O 2(g) 2 Cu. O(s) • Copper black • Copper (II) Oxide

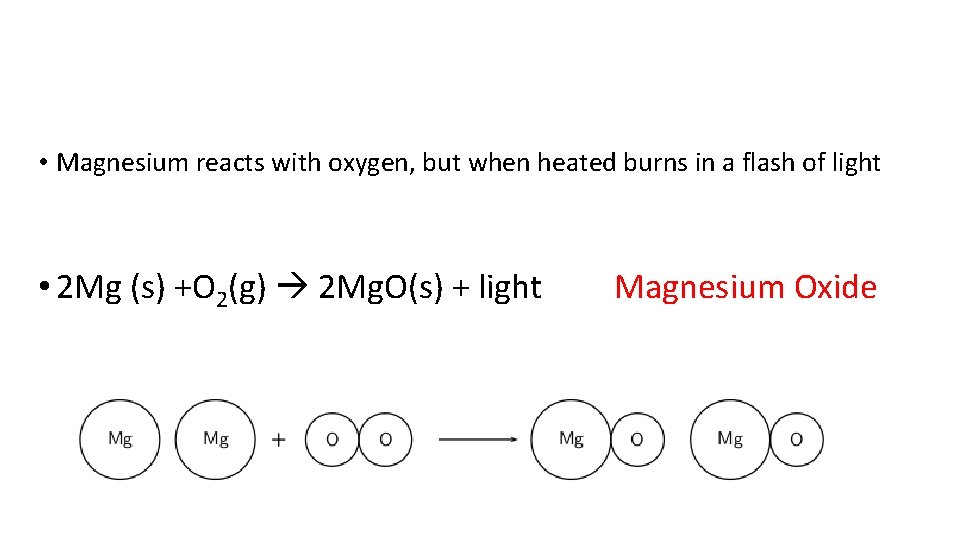
• Magnesium reacts with oxygen, but when heated burns in a flash of light • 2 Mg (s) +O 2(g) 2 Mg. O(s) + light Magnesium Oxide

• Gold will not react with oxygen, even when heated • Different metals have different levels of reactivity, just as you saw in the Relative Reactivities of Metals Lab • How would you rank the reactivities of Cu, Mg and Au? • Most Mg, Cu, Au least

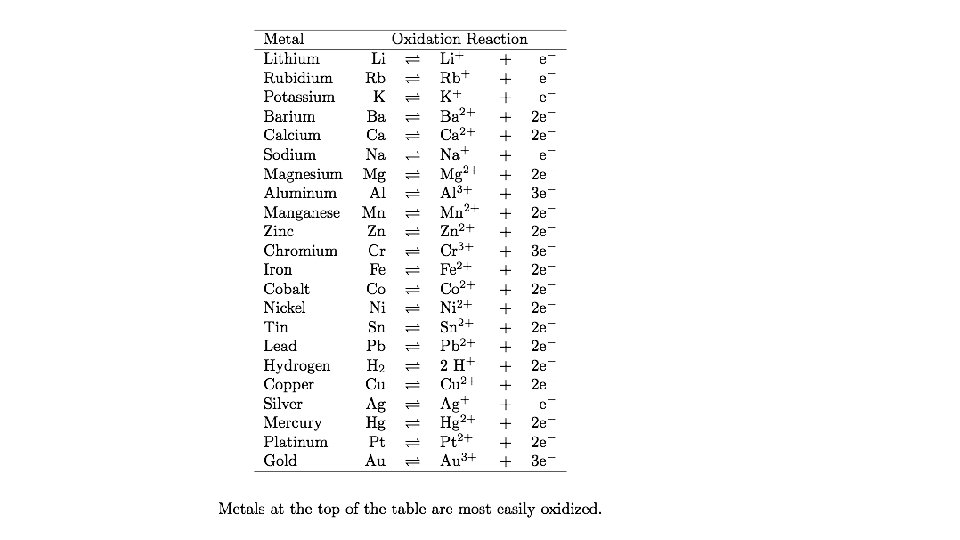
• Activity series • Ranking the elements according to their chemical reactivities • See handout • The activity series predicts if chemical reactions will occur • Most reactive at top • Least reactive at bottom


How do we know if a reaction will occur? • By looking at relative reactivities on the Metal Activity Series. • 1. Identify if the reaction will occur. 2. Predict the Products. • 1. K + Ag 2 S • • • 2. Br 2 + Na. Cl • • • 3. Pb (IV) + Ni. Cl 2

• Use your activity series and periodic table to answer the following questions 1. Will Pb metal replace Ag+ ions? 1. Yes, Pb is above Ag, it is more reactive

• 2. What trend in metallic reactivity is found as you move from left to right across a horizontal row (period) of the periodic table? (Hint: compare the reactivity of sodium with magnesium and aluminum. ) • Metal reactivity goes down as you move L to R

• 3. Where are the most reactive metals on the periodic table found? • Top left corner alkali and alkaline earth metals

• 4. Where are the least reactive metals located? • Transition Metals

• 5. Will iron (Fe) react with a solution of lead (II) nitrate (Pb(NO 3)2)? • Fe + PB(NO 3)2 yes, reactions happens, Fe is above Pb

• 6. Will platinum (Pt) metal react with a lead (II) nitrate solution? • Pt + Pb(NO 3)2 no, Pt is below Pb

• 7. Explain your answers to questions 5 and 6. • Element is above ion, reaction will happen • Element is below ion, reaction won’t happen

• Use specific examples from the activity series in your answers to these two questions: • Are the least reactive metals also the least expensive metals? • No, Au, Pt, Ag are least reactive but expensive.

• If not, what other factor(s) might influence the market value of a metal? • Abundance, fashion, preference, strength, how easily is it mined.

Examples from activity.
 Periodic trends in reactivity
Periodic trends in reactivity Trend in melting points of alkali metals
Trend in melting points of alkali metals How to learn reactivity series of metal
How to learn reactivity series of metal Facteur g
Facteur g Spraggins fitness
Spraggins fitness Acidity trends periodic table
Acidity trends periodic table Pure substance periodic table
Pure substance periodic table Nonmetals examples
Nonmetals examples Símbolo químico del hidrógeno
Símbolo químico del hidrógeno Diamond melting point
Diamond melting point When a metal reacts with a nonmetal the metal will
When a metal reacts with a nonmetal the metal will Solid liquid gas venn diagram
Solid liquid gas venn diagram El sodio es metal o no metal
El sodio es metal o no metal 0 25
0 25 Periodo y grupo
Periodo y grupo Metals vs nonmetals
Metals vs nonmetals Metals
Metals Chapter 12 basics of chemistry cosmetology
Chapter 12 basics of chemistry cosmetology