Periodic Trends Activity 3 PERIODIC TRENDS SUMMARY PERIODICITY














- Slides: 17

Periodic Trends Activity 3 PERIODIC TRENDS SUMMARY, PERIODICITY PRACTICE

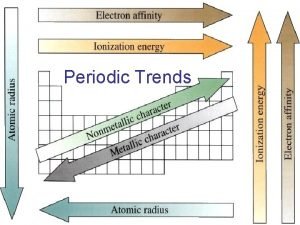
Objectives Today I will be able to: Analyze how the trends of atomic radius, ionization energy and electronegativity change across a period and down a family Compare the atomic radius, ionization energy and electronegativity of 2 or more elements Informal assessment: monitoring student questions and interactions as we complete the practice Formal assessment: analyzing the students interpretations of the trends, practice and exit ticket Common Core Connection Model with mathematics Look for and express regularity in repeated reasoning

Lesson Sequence Warm – Up Periodic Trends Summary Why do the trends occur notes Periodicity Practice Worksheet Applying the trends worksheet Exit Ticket

Warm - Up How does the atomic radius change across a period and down a family? How does electronegativity/ionization energy change across a period and down a family?

Objective Today I will be able to: Analyze how the trends of atomic radius, ionization energy and electronegativity change across a period and down a family Compare the atomic radius, ionization energy and electronegativity of 2 or more elements

Homework STEM Fair Corrections Due Friday 11/15 Finish Analyzing the Trends Worksheet

Agenda Warm-Up Finish Summary Why do the trends occur notes? Periodicity Practice Analyzing the trends Worksheet Exit Ticket

Take out part IV of the periodic trends activity and finish your summary COMPLETE YOUR SUMMARY (5 MINUTES)

WHY DO THE TRENDS OCCUR?

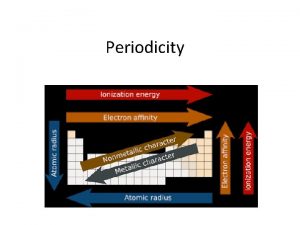
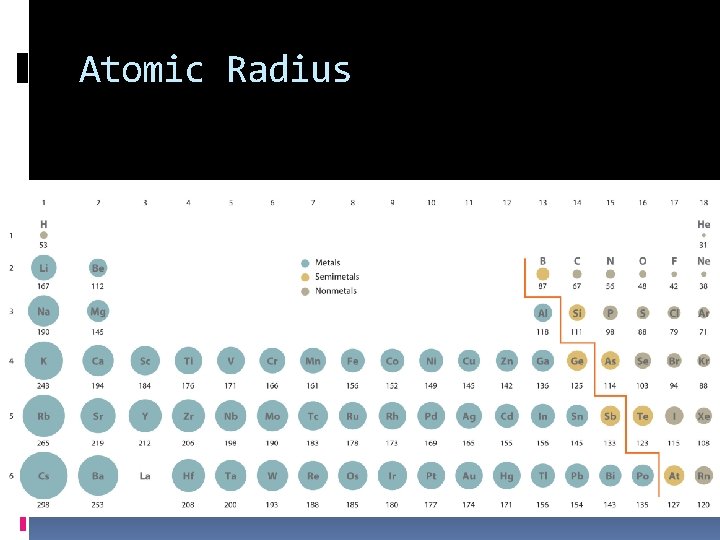
Atomic Radius… Decreases across a period Why? More protons in the nucleus, pulling the electron cloud closer to the nucleus Increases down a family Why? More energy levels for the electrons to fill

Atomic Radius

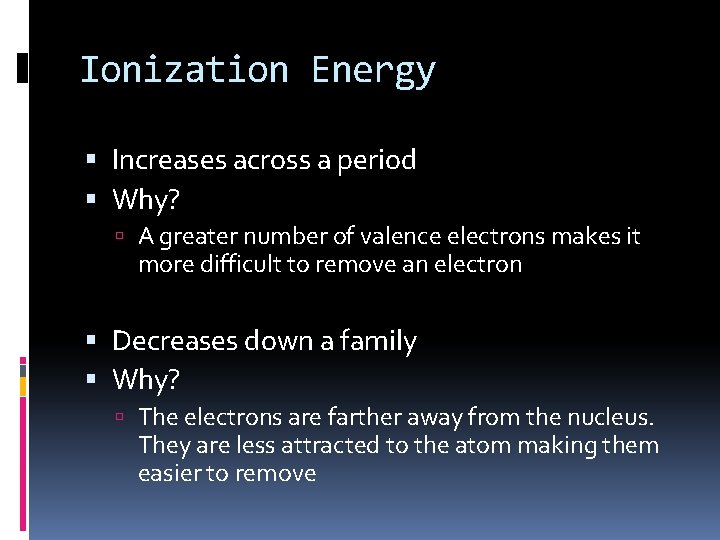
Ionization Energy Increases across a period Why? A greater number of valence electrons makes it more difficult to remove an electron Decreases down a family Why? The electrons are farther away from the nucleus. They are less attracted to the atom making them easier to remove

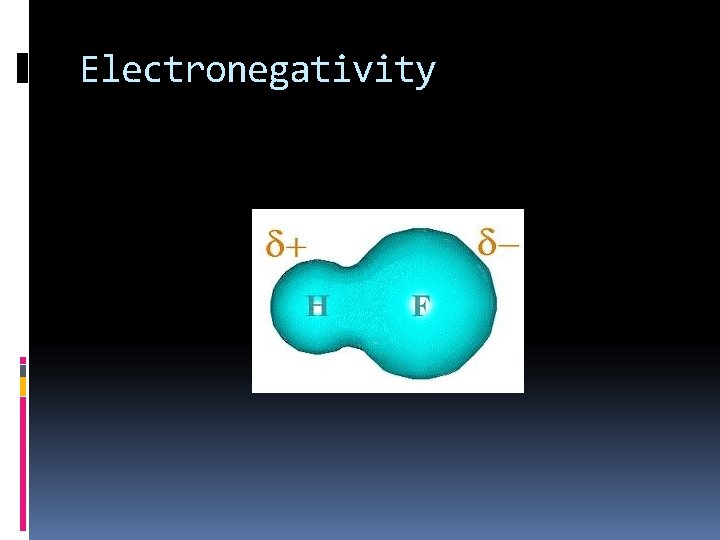
Electronegativity Increases across a period Why? Atoms are closer to reaching stability so they want to attract electrons Decreases down a family Why? The electrons are farther away from the nucleus in higher energy levels. They are not attracted to the nucleus as easily

Electronegativity

Complete the worksheet using your notes. Ask Ms. Ose for help if you have questions we will review selected problems. PERIODICITY PRACTICE WORKSHEET

Complete the analyzing the trends worksheet. If you do not finish in class, it will become homework. Be sure to respond to all parts of the question ANALYZING THE TRENDS WORKSHEET

Exit Ticket For the following set of elements: Ba Mg O Circle the element that has the smallest atomic radius Underline the element with the smallest ionization energy Box the element with the greatest electronegativity value
 Perioidic trends
Perioidic trends How to find out group and period of an element
How to find out group and period of an element Periodic trends activity worksheet
Periodic trends activity worksheet Summary of periodic trends
Summary of periodic trends What is periodicity?
What is periodicity? First dental home certification
First dental home certification Feeding periodicity adalah
Feeding periodicity adalah Chemsheets periodicity
Chemsheets periodicity Chapter 8
Chapter 8 Orbital diagram for k
Orbital diagram for k Ap chemistry chapter 7
Ap chemistry chapter 7 Aap bright futures periodicity schedule
Aap bright futures periodicity schedule Oxygen periodic trends
Oxygen periodic trends Atomic radius electronegativity
Atomic radius electronegativity Lymphatic filariasis
Lymphatic filariasis Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity Ionic radius trend
Ionic radius trend Electronegativity trend
Electronegativity trend