PERIODIC TRENDS PERIODIC TRENDS Periodic trends are patterns





- Slides: 15

PERIODIC TRENDS

PERIODIC TRENDS Periodic trends are patterns that are seen throughout the Periodic Table due to the way the elements are arranged. Trends occur both in groups and in periods.

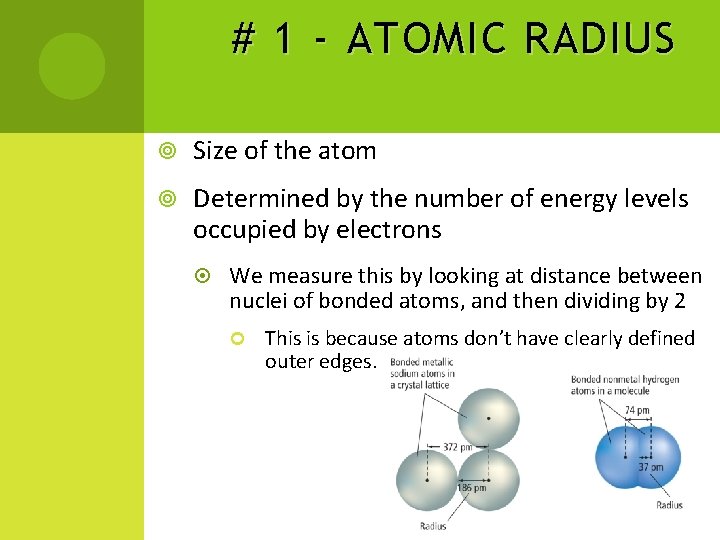
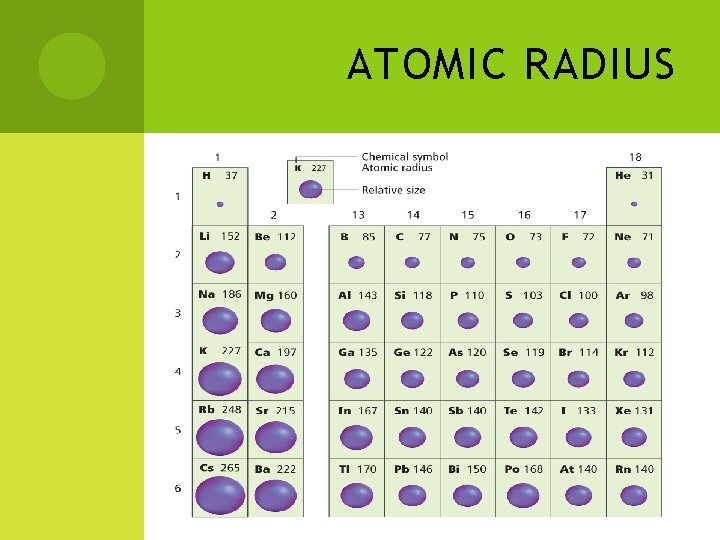
# 1 - ATOMIC RADIUS Size of the atom Determined by the number of energy levels occupied by electrons We measure this by looking at distance between nuclei of bonded atoms, and then dividing by 2 This is because atoms don’t have clearly defined outer edges.

ATOMIC RADIUS CONTINUED Trends: Groups trends – atomic size increases as you move down a group Number of energy levels containing electrons is increasing Period trends – atomic size decreases as you move from left to right across a period (within the same energy level) Increased attraction between the nucleus and the electrons (pulls electrons in tighter).

ATOMIC RADIUS

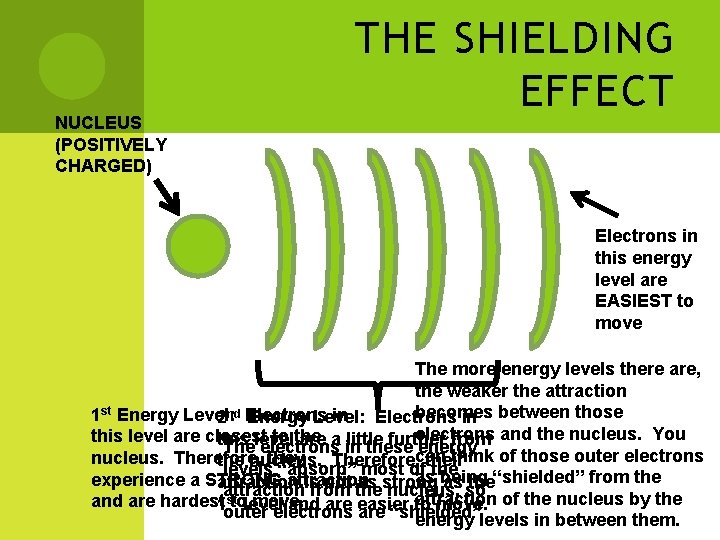
THE SHIELDING EFFECT Shielding Effect: Attraction between electrons & nucleus As more electrons are added (energy levels), less attraction to nucleus

NUCLEUS (POSITIVELY CHARGED) THE SHIELDING EFFECT Electrons in this energy level are EASIEST to move The more energy levels there are, the weaker the attraction becomes 1 st Energy Level: in Electrons 2 nd Electrons Energy Level: in between those electrons and the nucleus. You this level are closest to the this level are a little further from The electrons in these can energy think of those outer electrons nucleus. Therefore, they the nucleus. Therefore, their levels “absorb” most of the as being “shielded” from the experience a STRONG attraction is not as strong as the attraction from the nucleus, so of the nucleus by the attraction and are hardest 1 sttolevel move. and are easier to move. outer electrons are “shielded” energy levels in between them.

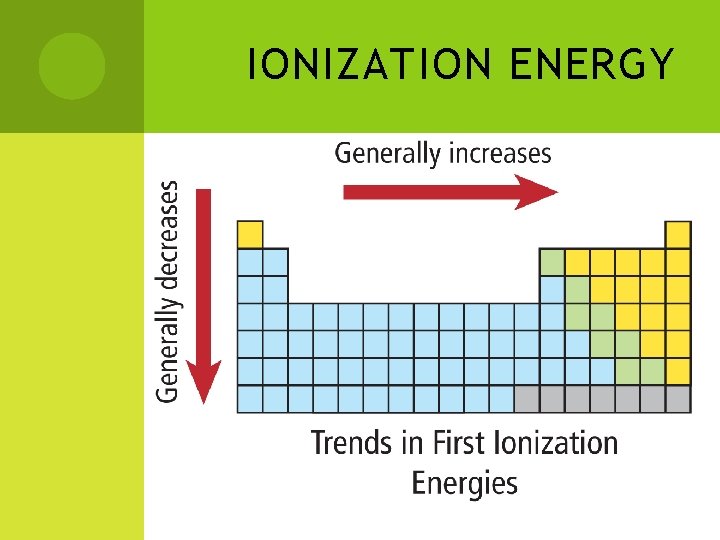
#2 - IONIZATION ENERGY Remember: Ions are atoms with charges (more or less electrons than protons. ) Ionization Energy is the energy needed to remove an electron thus creating an ion (charged particle) Energy is required in this process to overcome the attraction of the nucleus for the electrons The lower the energy, the easier it is to remove an electron Group trend - This energy decreases as you move down a group The valence electrons are further away from the nucleus Period trend - This energy increases across a period The atoms are getting slightly smaller. Therefore, electrons get closer to nucleus and thus the nucleus has a greater attraction on the electrons

IONIZATION ENERGY

# 3 - ELECTRON AFFINITY Energy change that results when an electron is added to an atom or ion In other words, it’s the ability of an atom to attract and hold an extra electron We measure Electron Affinity by the change in energy that occurs when an electron is added to an atom or ion

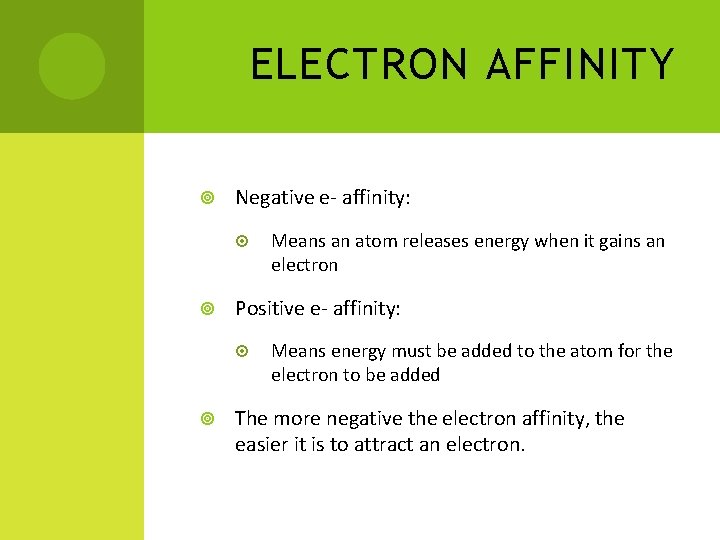
ELECTRON AFFINITY Negative e- affinity: Positive e- affinity: Means an atom releases energy when it gains an electron Means energy must be added to the atom for the electron to be added The more negative the electron affinity, the easier it is to attract an electron.

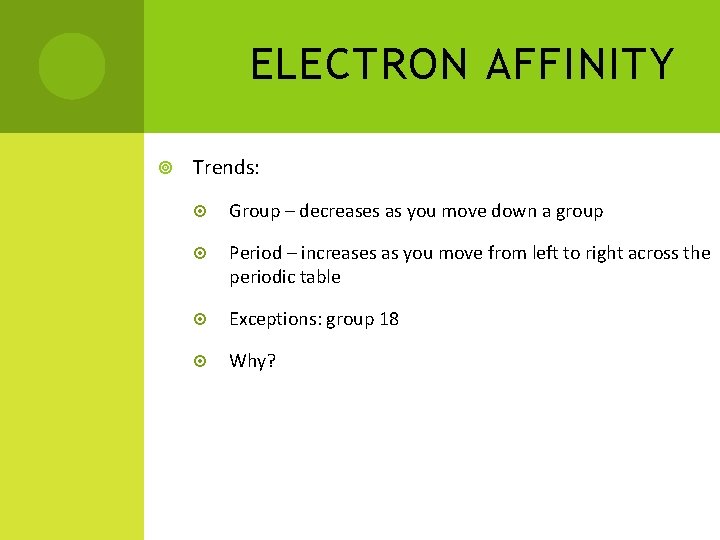
ELECTRON AFFINITY Trends: Group – decreases as you move down a group Period – increases as you move from left to right across the periodic table Exceptions: group 18 Why?

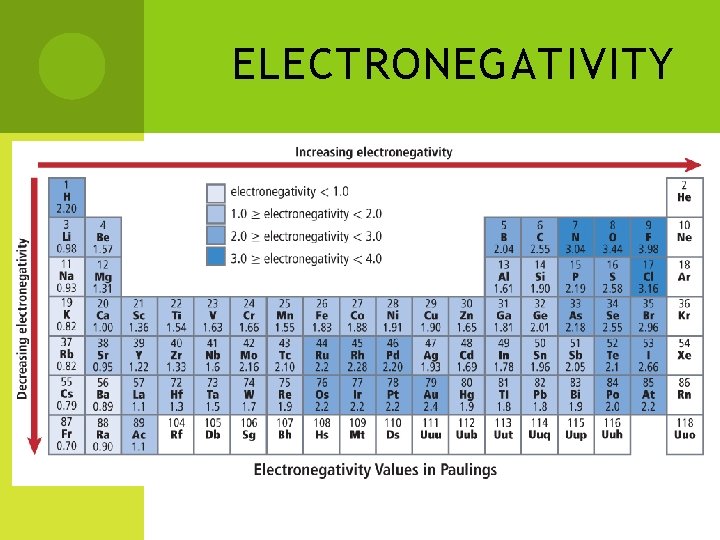
#4 ELECTRONEGATIVITY The tendency of an atom to attract eto itself when it is chemically combined with another element Electronegativity values range from 0. 7 to 4. 0 The larger the electronegativity value, the easier it is to attract an electron.

ELECTRONEGATIVITY CONTINUED Group trend - Values decrease going down a group Period trend - Values increase across a period As you go down a group, more energy levels are added. Therefore, the nucleus’ ability to attract electrons from other atoms weakens. The positive charge of the nucleus gets bigger due to increase in atomic number. Therefore, it has a stronger “pull” on electrons. Exception – group 18 is not included Why?

ELECTRONEGATIVITY