Periodic Oxide Trends YEAR 11 DP CHEMISTRY Nonmetal



- Slides: 7

Periodic Oxide Trends YEAR 11 DP CHEMISTRY

Non-metal Oxides Sources Atmospheric O 2 is very reactive and reacts with many substances to form oxides Natural formation 1. 2. 3. CO 2 – from respiration (“burning” sugars for energy) NO 2 – from lightning strikes (N 2 + 2 O 2 in the air 2 NO 2) SO 2 – released from volcanoes or H 2 S + O 2 SO 2 + H 2 O (H 2 S produced by bacterial decomposition of organic matter) Human causes (bushfires and burning fossil fuels) 3. 1. 2. 3. 4. 5. CO 2 – fossil fuel combustion product NO – high temperature combustion product NO 2 – NO is easily oxidised in the air (NO + O 2 NO 2) SO 2 – burning coal that contains S as an impurity SO 3 – SO 2 is easily oxidised in the air

Acidic Non-metal Oxides Many non-metal oxides react with water in the atmosphere to produce acids; CO 2 + H 2 O H 2 CO 3 (carbonic acid) SO 2 + H 2 O H 2 SO 3 (sulfurous acid) SO 3 + H 2 O H 2 SO 4 (sulfuric acid) 2 NO 2 + H 2 O HNO 3 + HNO 2 (nitric and nitrous acid) These non-metal oxides are all gases Their acidic products all contribute to the acidity of rain

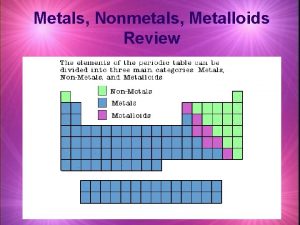
Oxide Trends in the Periodic Table Oxides tend to increase in acidity from left to right In general: �Metal oxides are basic (left side) �Non-metal oxides are acidic (right side) Exceptions: �Amphoteric oxides (i. e. Al, Be, Ga, Sn, Pb) Why this trend? This is due to electronegativity increasing from left to right (see following slides for more details)

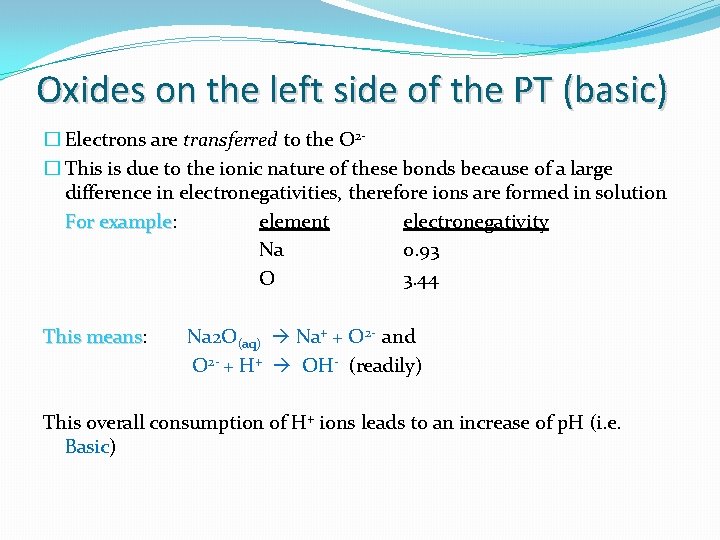
Oxides on the left side of the PT (basic) � Electrons are transferred to the O 2� This is due to the ionic nature of these bonds because of a large difference in electronegativities, therefore ions are formed in solution For example: element electronegativity example Na 0. 93 O 3. 44 This means: means Na 2 O(aq) Na+ + O 2 - and O 2 - + H+ OH- (readily) This overall consumption of H+ ions leads to an increase of p. H (i. e. Basic)

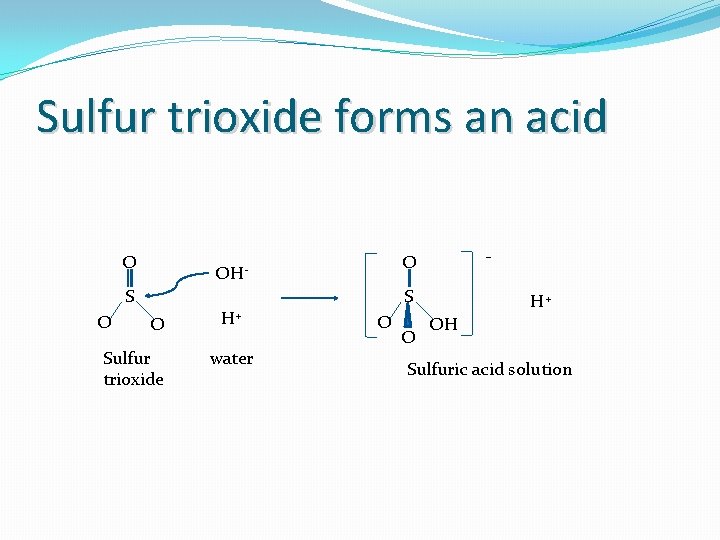
Oxides on the right side of the PT (acidic) � Electrons are shared with the O 2� This is due to the covalent nature of these bonds because of a small difference in electronegativities, thus no ions are formed For example: element electronegativity example S 2. 58 O 3. 44 This means: means Due to a partially positive S central atom, SO 3(aq) + H 2 O H+ + HSO 4 - This overall production of H+ ions leads to an decrease of p. H (i. e. acidic). (See following slide for details of this reaction)

Sulfur trioxide forms an acid O S O - O OH- S O Sulfur trioxide H+ water O O H+ OH Sulfuric acid solution