Periodic Trends Periodic Trends Periodic Trends are trends






- Slides: 16

Periodic Trends

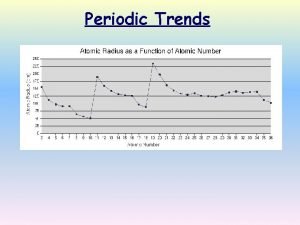
Periodic Trends • Periodic Trends are trends that occur across the periodic table and down the periodic table

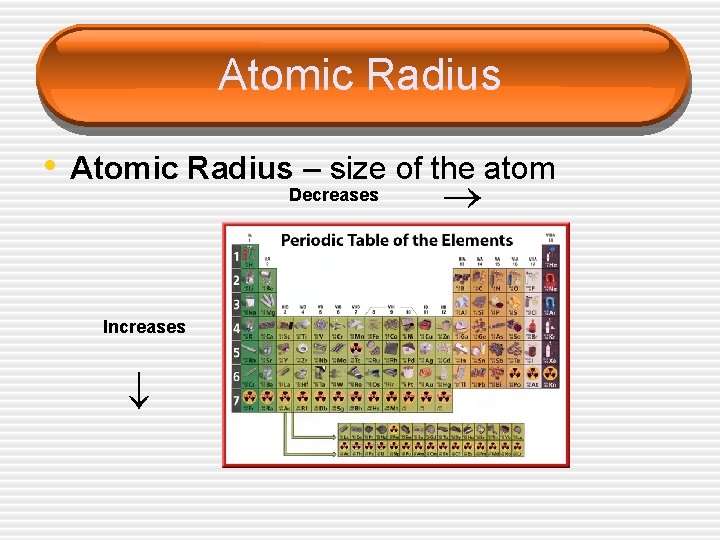
Atomic Radius Decreases Increases • Atomic Radius – size of the atom

Atomic Radius • Radius decreases across a period § Increased effective nuclear charge due to decreased shielding • Radius increases down a group § Addition of principal quantum levels

Ionization Energy • _________ – the energy required to Increases Decreases remove one mole of electrons, from one mole of gaseous atoms, to produce one mole of ions

Ionization Energy • First ionization energy • Second Ionization energy • Ionization energies are measure in KJ/mol • The have positive values showing that energy must be put in to remove an electron

Ionization Energy • The magnitude of the ionization energy depends on 2 things… § The nuclear charge (how many protons are present) § The shielding effect of the inner electrons

Ionization Energy • Across • increase because the nuclear charge increases and the electrons are being removed from the same principal quantum level (shell), experiencing no extra shielding, and are therefore held more strongly. • (You’re trying to remove a more & more negatives, but still have the same number of positives pulling in on the electrons. They are pulling harder because there are few negatives)

Ionization Energy • Down • decrease because the outer electrons are • further away from the nucleus Therefore the electrons are held less strongly.

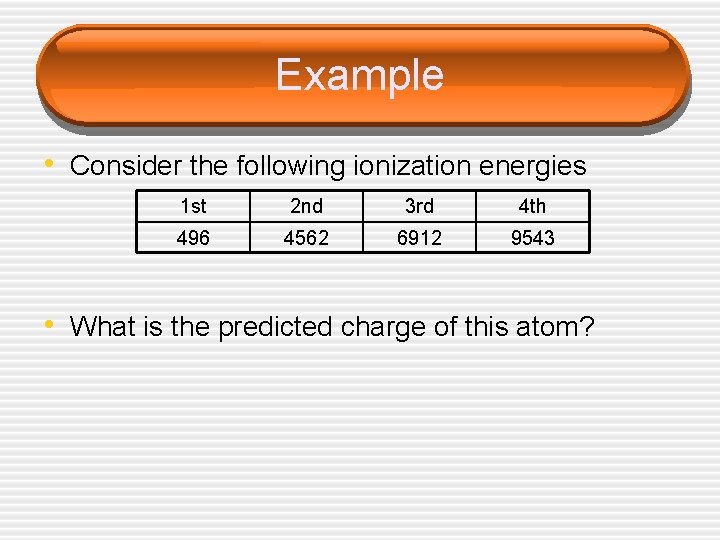
Example • Consider the following ionization energies 1 st 2 nd 3 rd 4 th 496 4562 6912 9543 • What is the predicted charge of this atom?

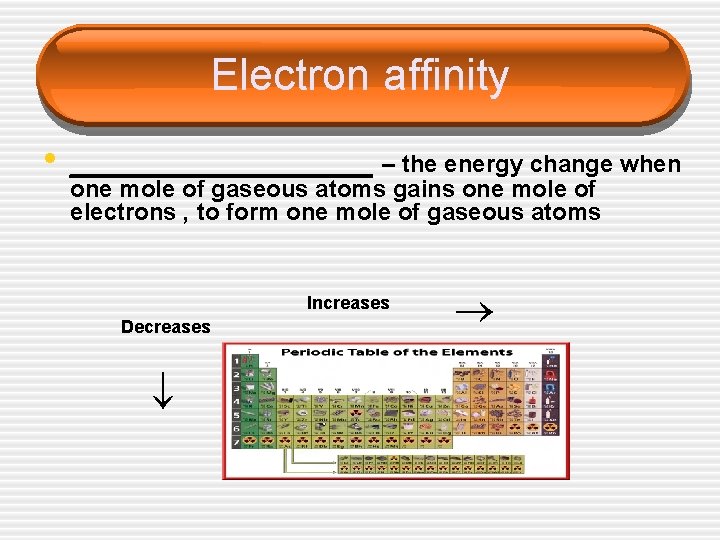
Electron affinity • _________ – the energy change when Increases Decreases one mole of gaseous atoms gains one mole of electrons , to form one mole of gaseous atoms

Electron affinity • Affinity tends to increase across a • period Affinity tends to decrease as you go down in a period § Electrons farther from the nucleus § experience less nuclear attraction

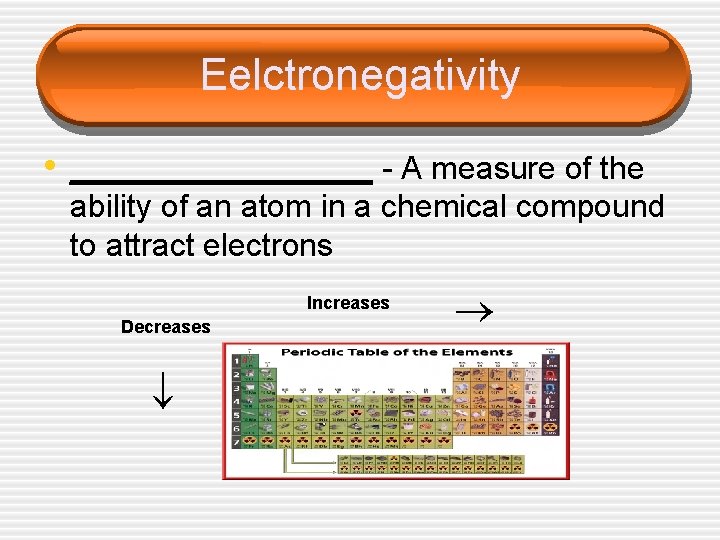
Eelctronegativity • _________ - A measure of the Increases Decreases ability of an atom in a chemical compound to attract electrons

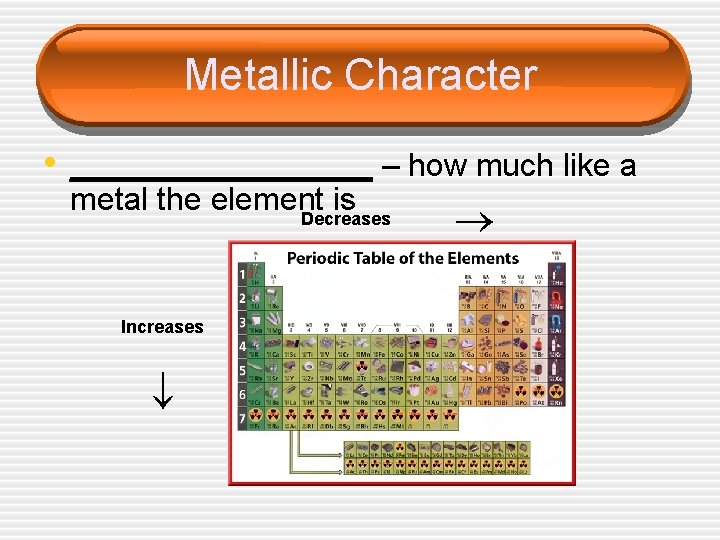
Metallic Character metal the element is Decreases Increases • _________ – how much like a

Ionic Radius • which will be larger: • Cl or Cl-1

Ionic Radius • which will be larger: • Na or Na+1
 Mikael ferm
Mikael ferm What are the trends in the periodic table
What are the trends in the periodic table Boron group number
Boron group number Summary of periodic trends
Summary of periodic trends Graphing periodic trends
Graphing periodic trends Electronegativity
Electronegativity Atomic radius trend
Atomic radius trend Periodic trends activity worksheet
Periodic trends activity worksheet Chemical bonding assignment
Chemical bonding assignment Trends of periodic table
Trends of periodic table Period trends
Period trends Coulomb's law periodic trends
Coulomb's law periodic trends Periodic trends in properties of elements
Periodic trends in properties of elements Oxidation trends periodic table
Oxidation trends periodic table Periodic trends in elemental properties
Periodic trends in elemental properties Atomic radius definition
Atomic radius definition Periodic trends
Periodic trends