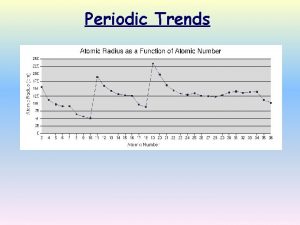
Periodic Trends Atomic Radius Half of the distance













- Slides: 27

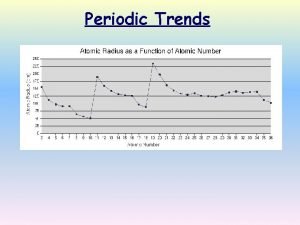
Periodic Trends

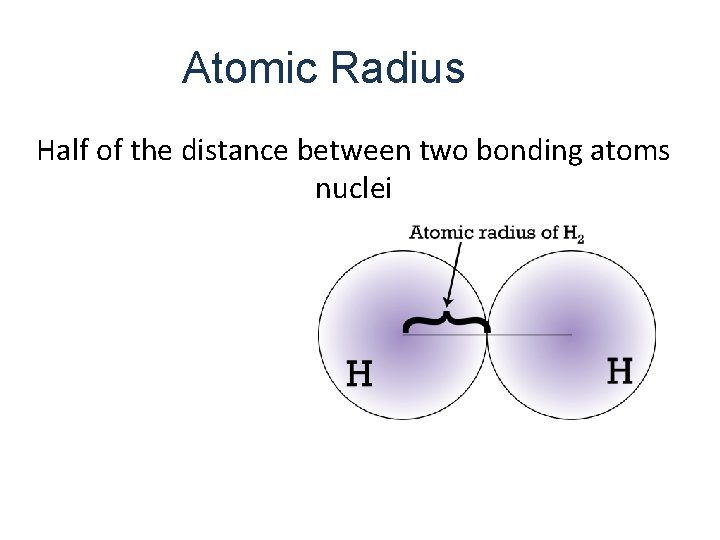
Atomic Radius Half of the distance between two bonding atoms nuclei

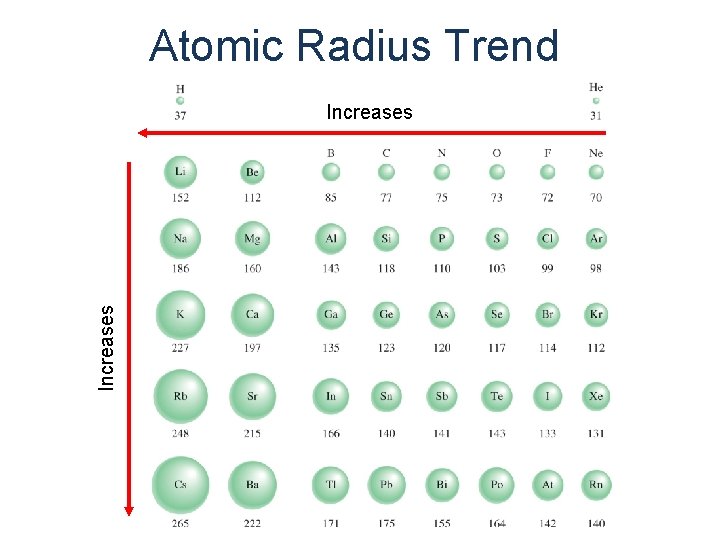
Atomic Radius Trend Increases

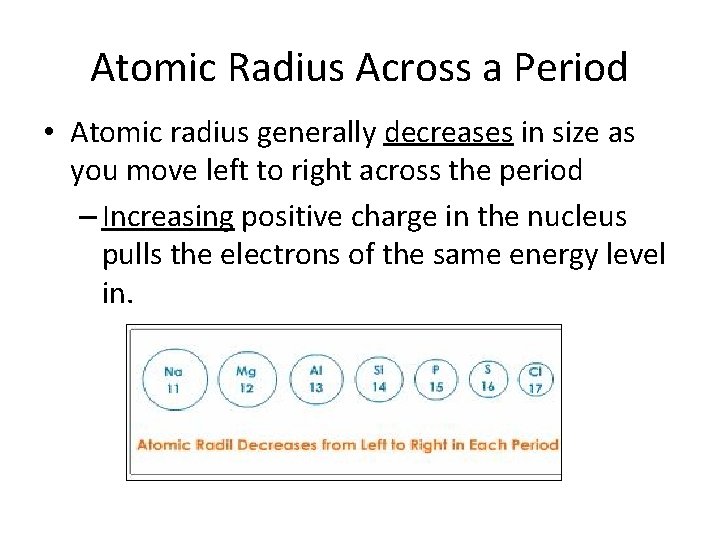
Atomic Radius Across a Period • Atomic radius generally decreases in size as you move left to right across the period – Increasing positive charge in the nucleus pulls the electrons of the same energy level in.

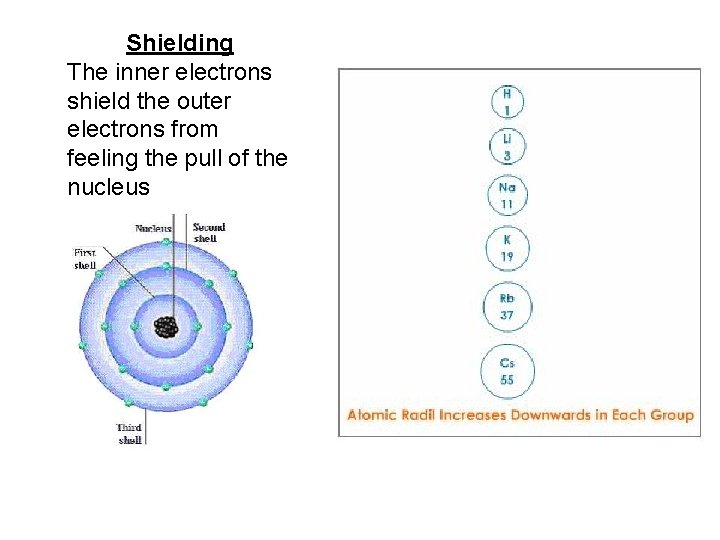
Atomic Radius Down a Group • Atomic radius increases as you move down a group – Orbital size increases as you move down a group with increasing energy level – Larger orbitals means that outer electrons are farther from the nucleus. This increased distance offsets the greater pull of the increased nuclear charge. – As additional orbitals between the nucleus and the outer electrons are occupied, the inner electrons shield the outer electrons from the pull of the nucleus this is called shielding.

Shielding The inner electrons shield the outer electrons from feeling the pull of the nucleus

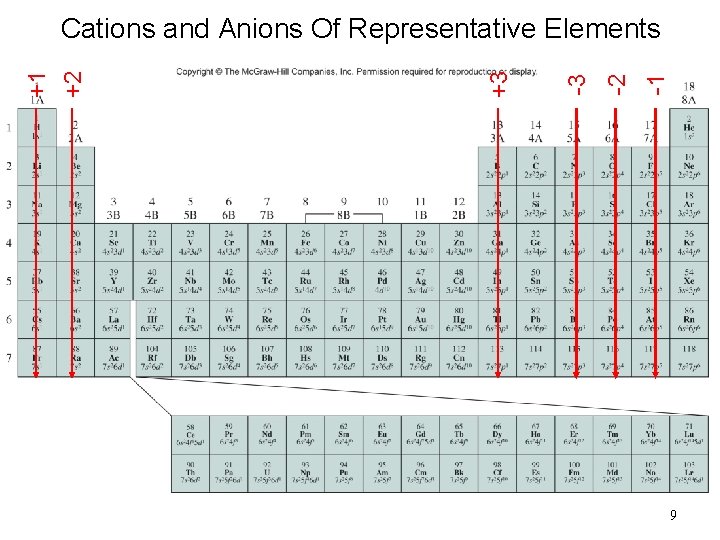
Ionic Radius • Compare ions to their neutral counterparts • Ion: charged atom that results from the gaining or losing of electrons • Cation: loses electrons and produces a positive charge • Anion: gains electrons and produces a negative charge

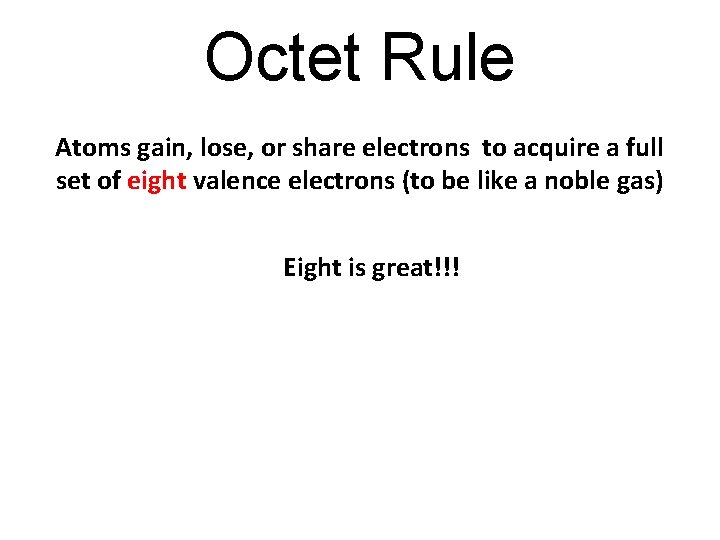
Octet Rule Atoms gain, lose, or share electrons to acquire a full set of eight valence electrons (to be like a noble gas) Eight is great!!!

-1 -2 -3 +3 +1 +2 Cations and Anions Of Representative Elements 9

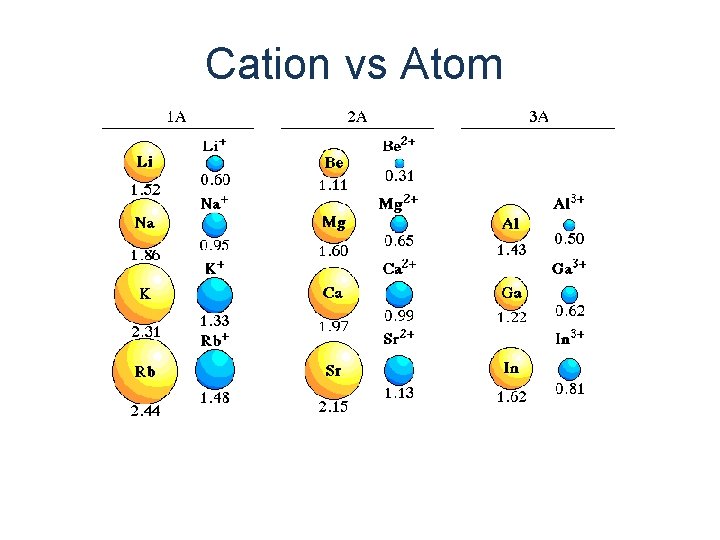
Cation vs Atom

Atom 12 electrons 12 protons Mg Magnesium Atom Cation 10 electrons 12 protons Mg 2+ Magnesium Cation

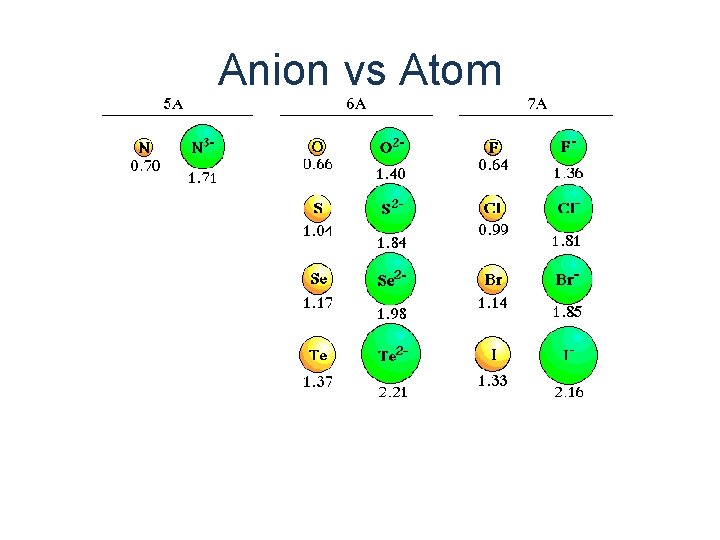
Anion vs Atom

Atom Anion 18 electrons 16 protons S Sulfur Atom 16 protons S 2 Sulfer Anion

Cation is always smaller than atom from which it is formed. Anion is always larger than atom from which it is formed.

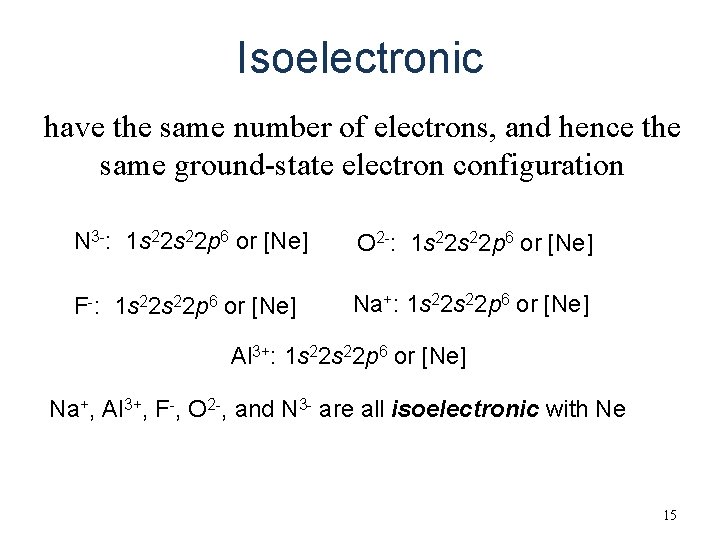
Isoelectronic have the same number of electrons, and hence the same ground-state electron configuration N 3 -: 1 s 22 p 6 or [Ne] O 2 -: 1 s 22 p 6 or [Ne] F-: 1 s 22 p 6 or [Ne] Na+: 1 s 22 p 6 or [Ne] Al 3+: 1 s 22 p 6 or [Ne] Na+, Al 3+, F-, O 2 -, and N 3 - are all isoelectronic with Ne 15

Periodic Table video

WUP#14 For the element Br find the following: 1. 2. 3. 4. Electron configuration Excited state Noble gas configuration Orbital diagram

Periodic Trends day 2

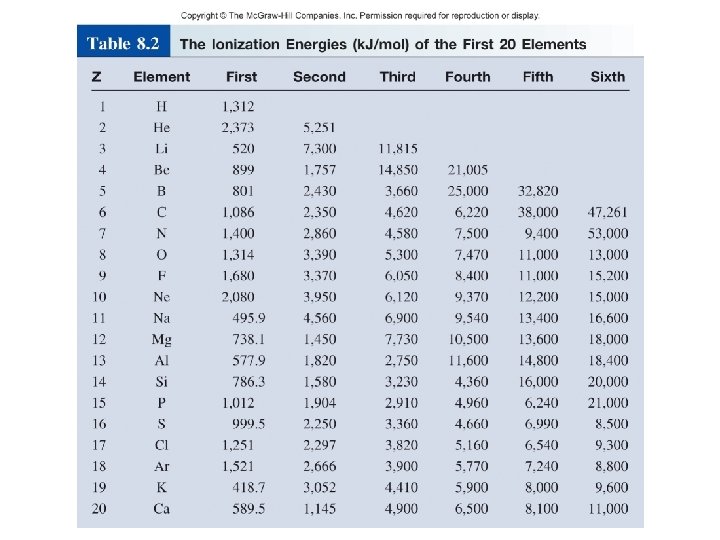
Ionization Energy • Minimum energy required to remove an electron from a gaseous atom in its ground state • Indication of how strongly an atom’s nucleus holds onto its valence electron


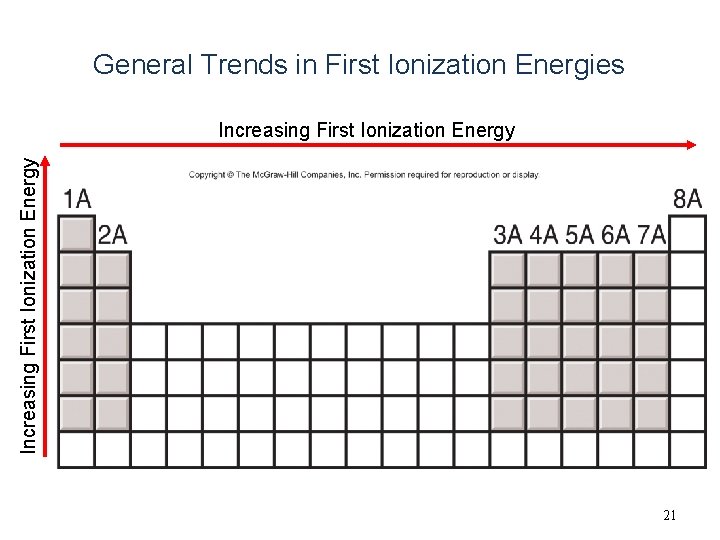
General Trends in First Ionization Energies Increasing First Ionization Energy 21

Ionization Energy Trends – Across a Period • Ionization energy generally increases as you move left to right, including noble gases – Across a period electrons are added to the same energy level (same distance away from the nucleus), yet the nuclear charge is increasing across a period increasing the attraction to the electrons.

Ionization Energy Trends – Down a Group • Ionization energy decreases as you move down a group – Down a group electrons are added to a higher energy level (farther distance away from the nucleus), making it easier to remove an electron

Electronegativity • Indicates an element’s ability to attract electrons in a shared chemical bond • fluorine (F) is the most electronegative element • cesium (Cs) and francium (Fr)are the least electronegative • Noble gases do not tend to have an electronegativity number since they tend not to form compounds


Electronegativity Memorize! F > O > Cl, N > Br > C, S > I > H , P Frank Owes Claire Nine Brownies Cause She Isa Hungry Person *elements w commas between are = E. N.

Trends with Electronegativity • Electronegativity increases as you move left-to -right across a period • Electronegativity decreases as you move down a group
 Atomic radius
Atomic radius Fr atomic radius
Fr atomic radius Ionization energy trend
Ionization energy trend Atomic number vs atomic radius
Atomic number vs atomic radius Periodic trends of elements
Periodic trends of elements Ionization energy trend periodic table
Ionization energy trend periodic table How to find out group and period of an element
How to find out group and period of an element Ionic radius trends
Ionic radius trends Nonbonding atomic radius
Nonbonding atomic radius Periodic trends definition
Periodic trends definition Which element has the greatest electronegativity
Which element has the greatest electronegativity Do metals have high ionization energy
Do metals have high ionization energy Periodic table radius
Periodic table radius Smallest atomic radius
Smallest atomic radius Mendeleev
Mendeleev Periodic trends definition
Periodic trends definition Largest atomic radius
Largest atomic radius Atomic radius summary
Atomic radius summary Atomic radius snowman
Atomic radius snowman Atomic radius across a period
Atomic radius across a period Smallest atomic radius
Smallest atomic radius Mayan periodic table
Mayan periodic table Atomic radius pattern
Atomic radius pattern Which atoms has the largest atomic radius
Which atoms has the largest atomic radius Atomic radius
Atomic radius Exothermic electron affinity trend
Exothermic electron affinity trend Formula for bohr radius
Formula for bohr radius Nuclear charge periodic trend
Nuclear charge periodic trend