Periodic Trends Atomic Radius Ionization Energy Electronegativity Metallic






- Slides: 18

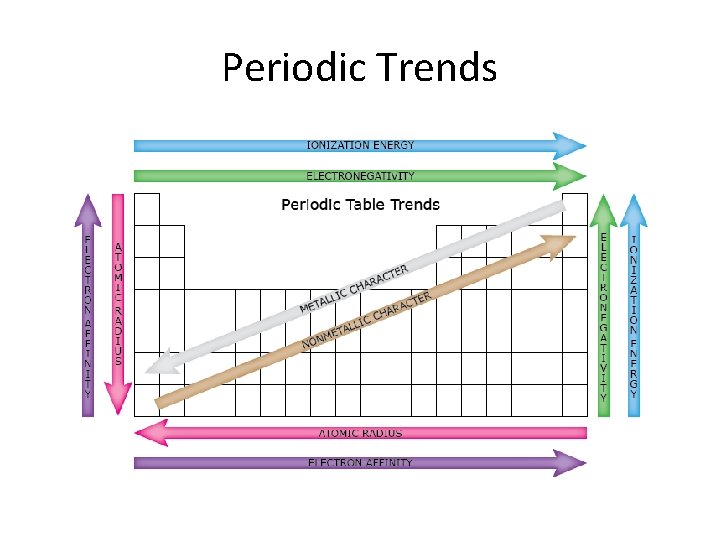
Periodic Trends: • Atomic Radius • Ionization Energy • Electronegativity • Metallic properties

Periodic trends & Valence electrons are the electrons found at the highest energy level and are available to form chemical bonds. We describe valence electrons using Lewis Dots.

Atomic Radius • Describes the size of an atom – Atomic radius decreases across the period and increases down the group.

1 st Ionization Energy • Describes the energy required to remove an electron from an atom. (forms cations) – Ionization energy increased across the period and decreases down the group.

Electronegativity • Describes an atoms ability to gain an electron. (forms anions) – Electronegativity increases across the period and decreases down the group.

Metallic Property • Metals are defined by their ability to give up electrons and form cations. – Metallic property decreases across the period and increases down the group.

Periodic Trends

Applying Periodic Trends: 1. Elements with 1 -3 valence electrons like to lose electrons to form cations. 2. Elements with 5 -7 valence electrons like to gain electrons to form anions. 3. All elements like to gain/lose or share electrons to have 8 valence electrons.

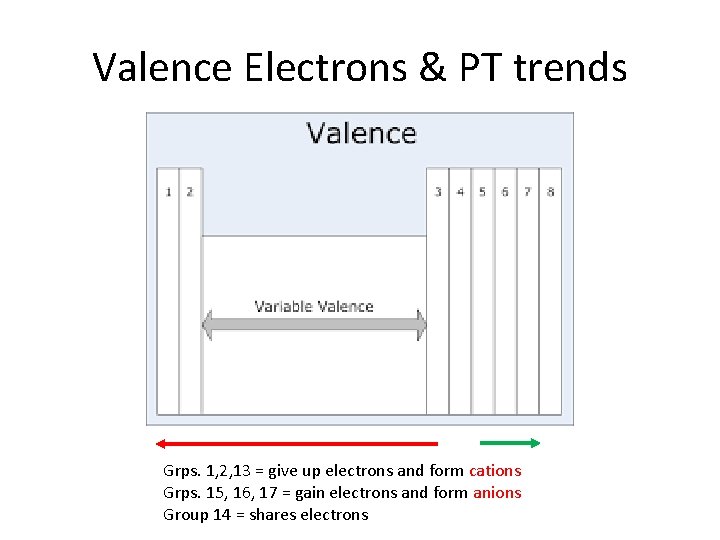
Valence Electrons & PT trends Grps. 1, 2, 13 = give up electrons and form cations Grps. 15, 16, 17 = gain electrons and form anions Group 14 = shares electrons

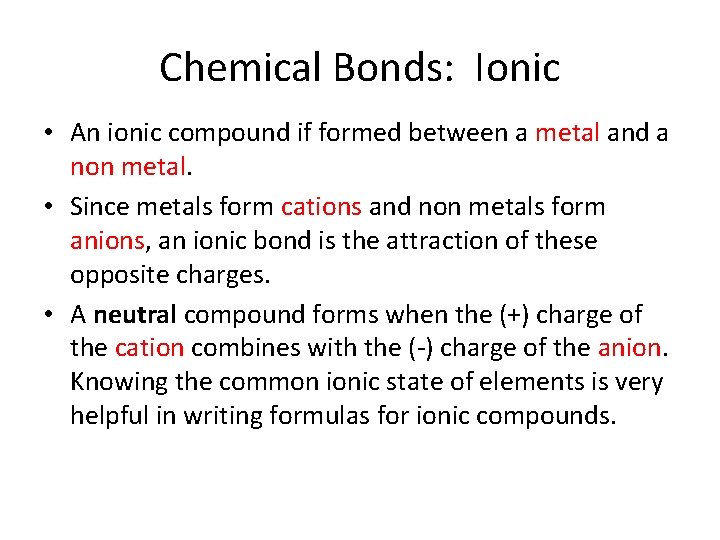
Chemical Bonds: Ionic • An ionic compound if formed between a metal and a non metal. • Since metals form cations and non metals form anions, an ionic bond is the attraction of these opposite charges. • A neutral compound forms when the (+) charge of the cation combines with the (-) charge of the anion. Knowing the common ionic state of elements is very helpful in writing formulas for ionic compounds.

Guidelines for writing/naming ionic compounds Name the following compound: Na. Cl Sodium chloride 1. 2. 3. 4. Always put the metal (cation) before the non metal (anion) The name of the metal stays the same The name of the non metal name changes: (-ide/ -ate, -ite) Identify the numerical charge of each ion and criss-cross the number to make a subscript. 5. When necessary, reduce the subscript to the lowest whole number ration.

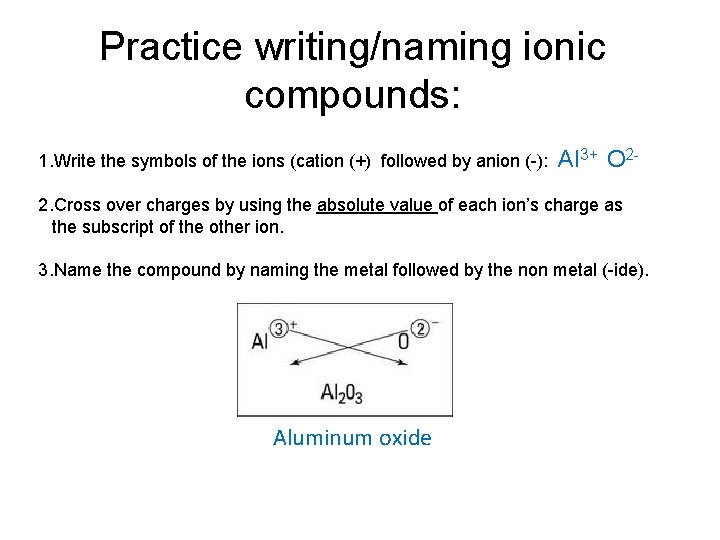
Practice writing/naming ionic compounds: 1. Write the symbols of the ions (cation (+) followed by anion (-): Al 3+ O 2 - 2. Cross over charges by using the absolute value of each ion’s charge as the subscript of the other ion. 3. Name the compound by naming the metal followed by the non metal (-ide). Aluminum oxide

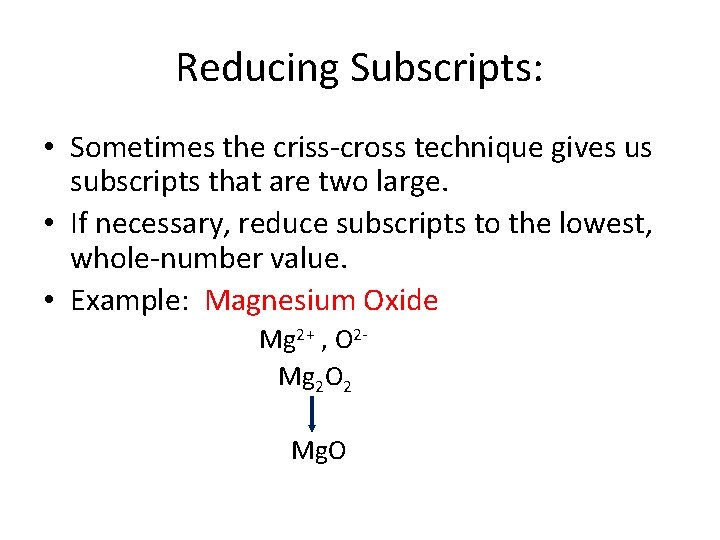
Reducing Subscripts: • Sometimes the criss-cross technique gives us subscripts that are two large. • If necessary, reduce subscripts to the lowest, whole-number value. • Example: Magnesium Oxide Mg 2+ , O 2 Mg 2 O 2 Mg. O

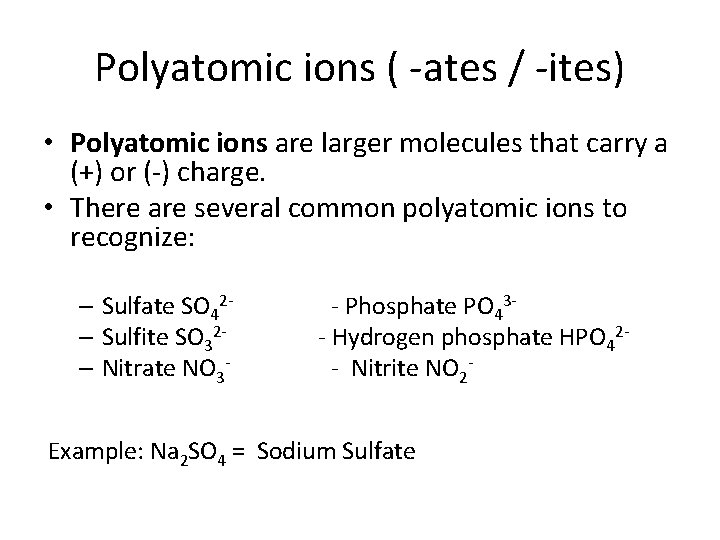
Polyatomic ions ( -ates / -ites) • Polyatomic ions are larger molecules that carry a (+) or (-) charge. • There are several common polyatomic ions to recognize: – Sulfate SO 42– Sulfite SO 32– Nitrate NO 3 - - Phosphate PO 43 - Hydrogen phosphate HPO 42 - Nitrite NO 2 - Example: Na 2 SO 4 = Sodium Sulfate

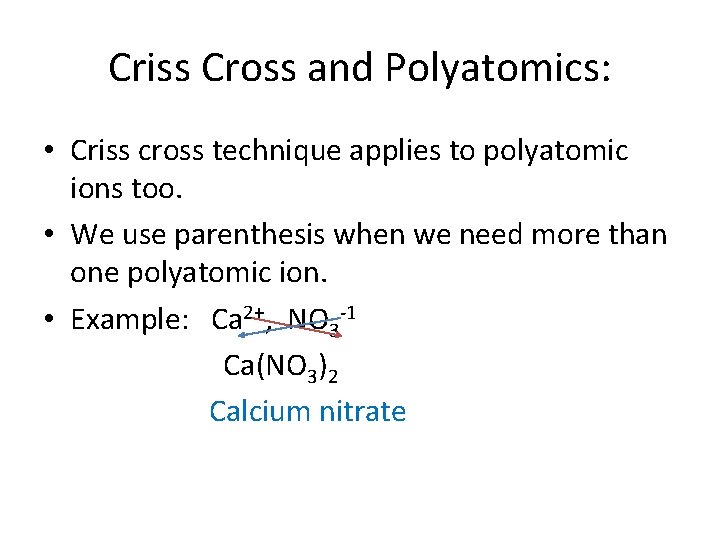
Criss Cross and Polyatomics: • Criss cross technique applies to polyatomic ions too. • We use parenthesis when we need more than one polyatomic ion. • Example: Ca 2+, NO 3 -1 Ca(NO 3)2 Calcium nitrate

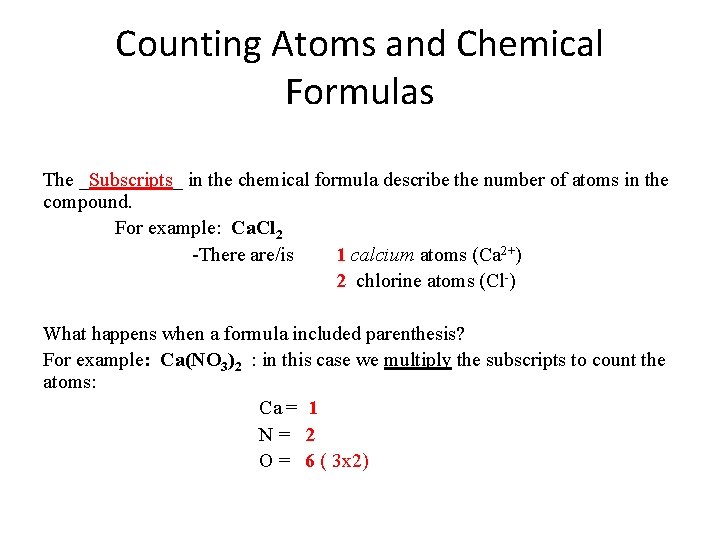
Counting Atoms and Chemical Formulas The _Subscripts_ in the chemical formula describe the number of atoms in the compound. For example: Ca. Cl 2 -There are/is 1 calcium atoms (Ca 2+) 2 chlorine atoms (Cl-) What happens when a formula included parenthesis? For example: Ca(NO 3)2 : in this case we multiply the subscripts to count the atoms: Ca = 1 N= 2 O = 6 ( 3 x 2)


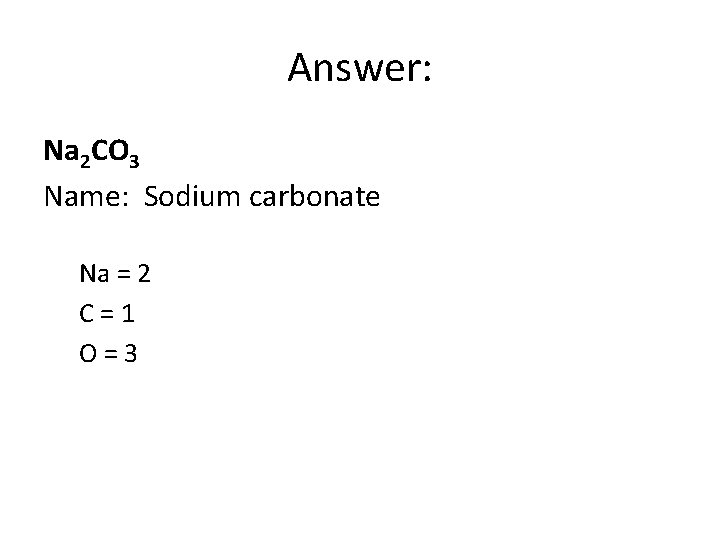
Answer: Na 2 CO 3 Name: Sodium carbonate Na = 2 C=1 O=3