W O H M A I Nonmetal halogen












- Slides: 17

W O H M A I?

• Non-metal, halogen family, atomic mass 35 § • Chlorine 25 electrons, transition element § Manganese • Gas, 48 neutrons § Krypton

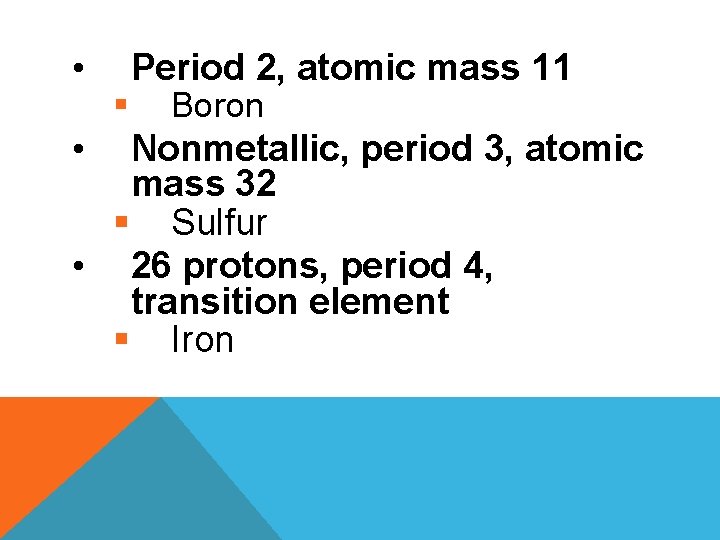
• • § Period 2, atomic mass 11 Boron Nonmetallic, period 3, atomic mass 32 § Sulfur • 26 protons, period 4, transition element § Iron

• 12 neutrons, metallic, 11 electrons § • Sodium 29 electrons, period 4 § Copper • Atomic mass 20, gas § Neon

• § • Period 5, transition element, 51 neutrons Zirconium 80 electrons, transition element § Mercury • Period 4, lowest mass on periodic tables § Potassium

• Metallic, period 4, 20 electrons § • Calcium Period 6, gas, 86 proton § Radon • 4 neutrons, metallic § Lithium

• Period 4, metallic, 27 electrons § • Cobalt Metallic, period 6, 56 protons § Barium • Gas, atomic mass 16, 8 neutrons § Oxygen

• Mass less than 30, not neon, noble gas § • Helium Period 5, metallic, 38 electrons § Strontium

Y R A T N E L E M E F C A S T

• Vertical columns in the period table • Families • Elements in families have similar _____ • Properties

• Family of “salt-producing” elements like the non-metal in table salt • Halogens • Family in Group 18 on periodic table • Noble Gas

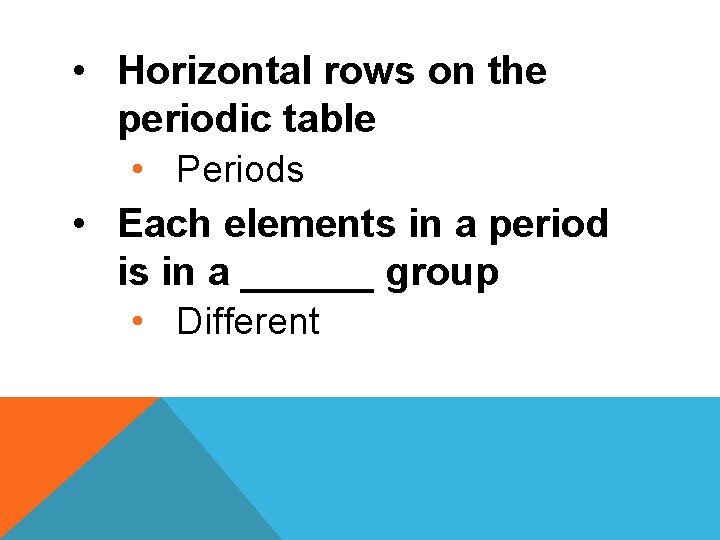
• Horizontal rows on the periodic table • Periods • Each elements in a period is in a ______ group • Different

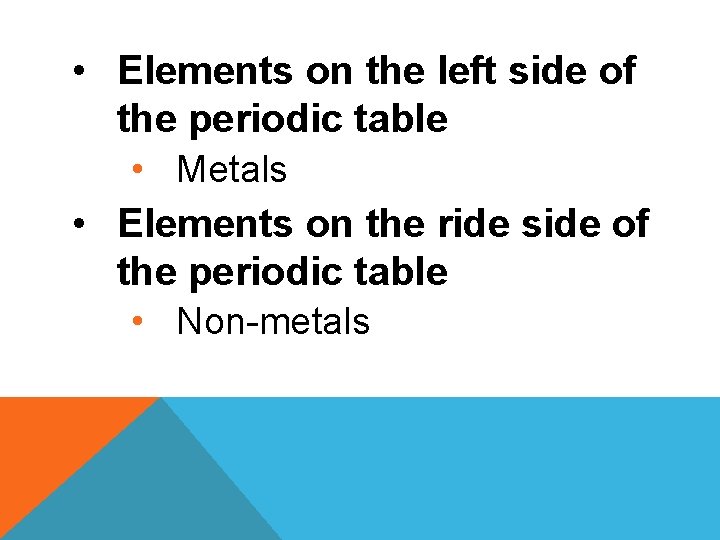
• Elements on the left side of the periodic table • Metals • Elements on the ride side of the periodic table • Non-metals

• Elements in Groups 3 -12 on the periodic table • Transitions • Most widely used metal • Iron

• Only liquid metal at room temperature • Mercury • Most abundant element in Earth’s crust • Aluminum

• Odorless, tasteless, colorless gas; lightest of all elements • Hydrogen • Second most abundant element in Earth’s crust; found in glass and sand • Silicon

• Gas element safe to use in balloons to make them float • Helium • Element contained in 80% of known compounds • Carbon