Periodic Trends Part III Ionization Energy Trends Electronegativity













- Slides: 25

Periodic Trends Part III: Ionization Energy Trends Electronegativity Trends

Which should have the LARGER radius? Cs+ or Li+ Mg 2+ or F O 2 - or N 3 K+ or Ti 2+

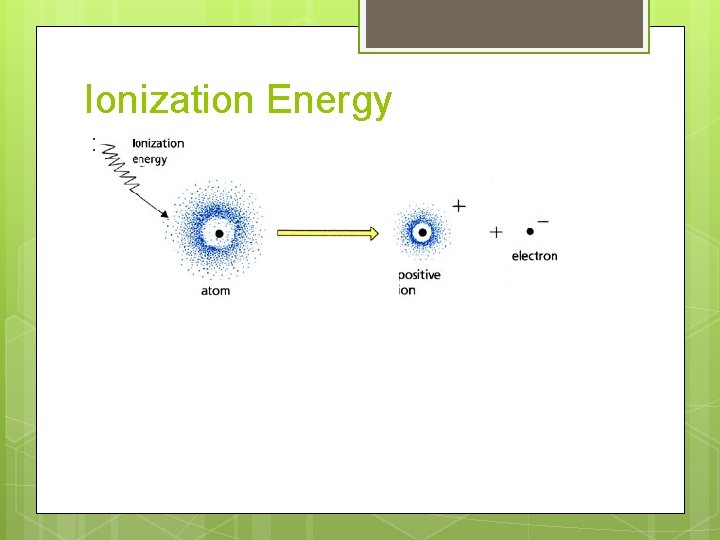
Ionization Energy :

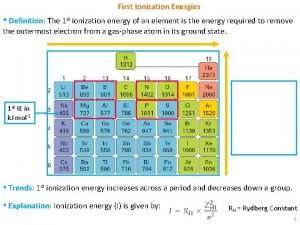
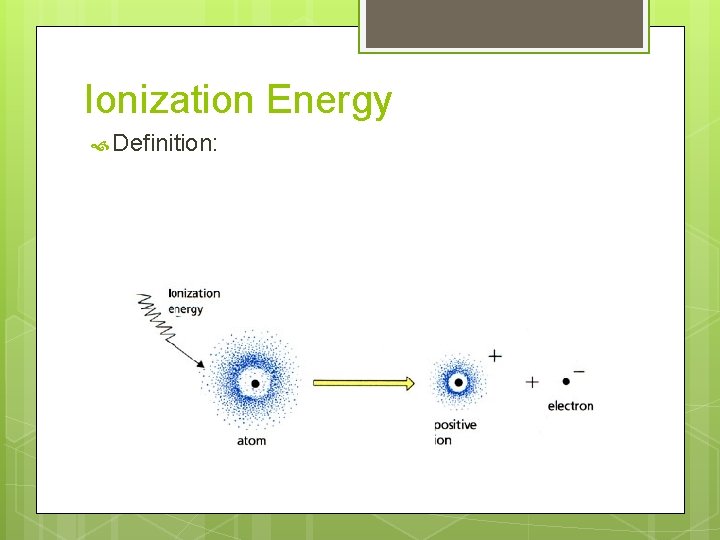
Ionization Energy Definition:

Patterns What elements tend to lose electrons?

Patterns What elements tend to gain electrons?

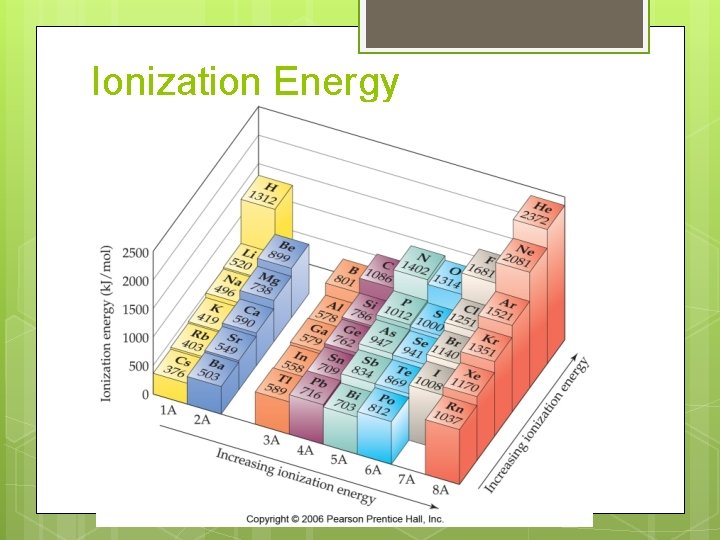
Ionization Energy

Ionization Energy trends Period trend Group trend

Ionization Energy First Ionization Energy Second Ionization Energy

Multiple ionization energies IE 1 IE 2 2 nd ionization energy is usually much larger than the 1 st ionization energy Atoms/ions don’t easily disrupt noble-gas like electron configurations

Electronegativity Definition:

Electronegativity Most electronegative element: Least electronegative element:

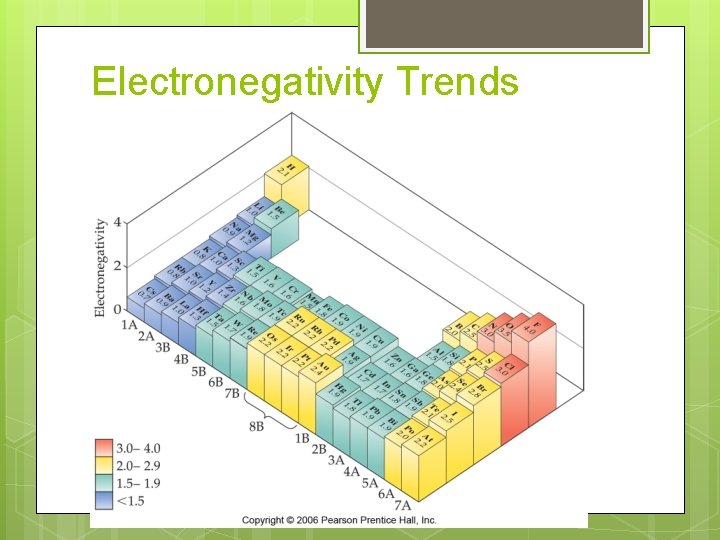
Electronegativity Trends

Electronegativity Period Trend: Group Trend:

Exceptional Electron Configurations Some elements have electron configurations that don’t follow the arrow-filling diagram Ex. Cu Expected: Actual:

Exceptional Electron Configurations Some elements have electron configurations that don’t follow the arrow-filling diagram Why? Minimize electron-electron repulsions with symmetric, spherical electron clouds half-filled or fully filled sublevels

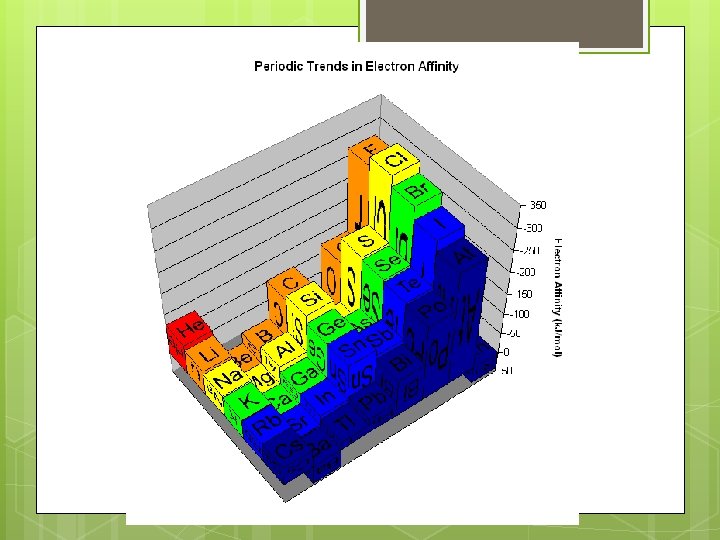
Electron Affinity Note: your book uses a different convention…do not read about EA in your textbook! The energy change that occurs when an electron is added to an ISOLATED (gaseous) atom How easily does an atom gain an electron?

Electron affinity The greater the attraction between an atom and an added electron, the more energy is given off…more negative EA


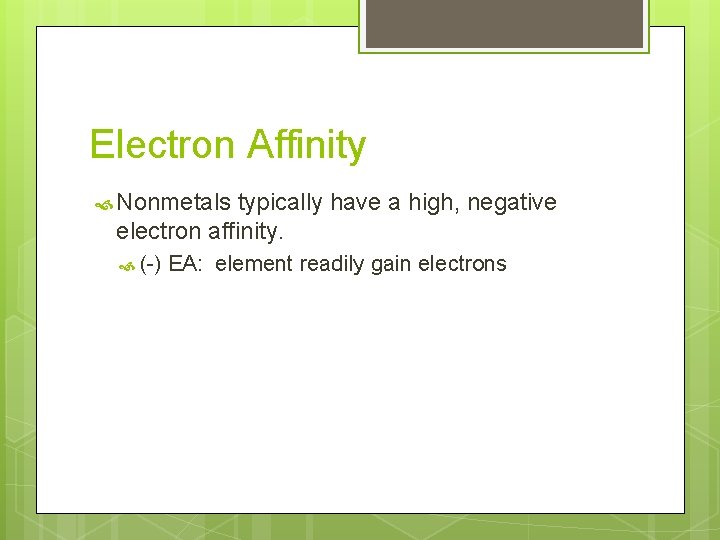
Electron Affinity Nonmetals typically have a high, negative electron affinity. (-) EA: element readily gain electrons

Electron Affinity Metals typically have a low electron affinity. Slightly negative or (+) EA: putting an electron in a higher energy sublevel is energetically unfavorable

Electron affinity Group trend: Electron affinity DECREASES slightly down a column Electron is added to an energy level farther from the nucleus, so the coulombic attraction is weaker

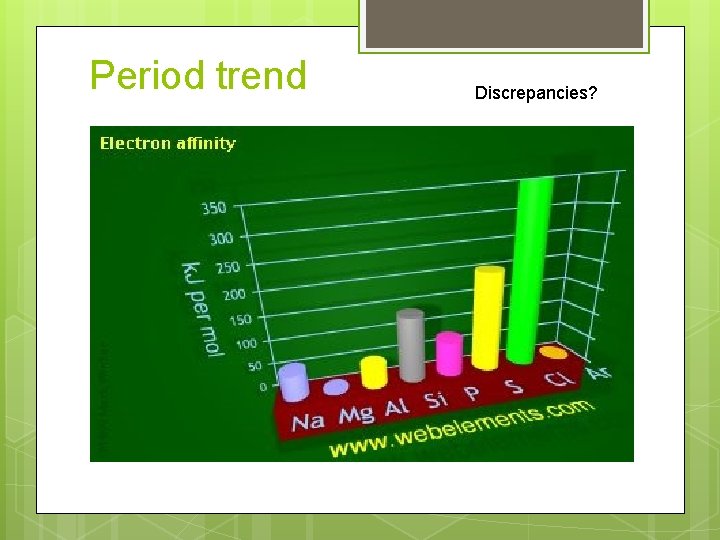
Period trend Discrepancies?

Electron affinity Period trend: Electron affinity generally INCREASES L R across a period Discrepancies Group 2, noble gases: low or positive electron affinity Place additional electron in a higher energy level

Electron affinity Period trend: Electron affinity generally INCREASES L R across a period Discrepancies Group 15: an additional electron would have to share an orbital…More electron-electron repulsions
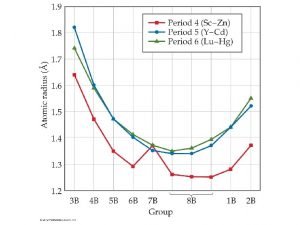
 Period trends
Period trends Atomic radius in periodic table
Atomic radius in periodic table Electronegativity vs ionization energy
Electronegativity vs ionization energy Periodic table trends
Periodic table trends Periodic trends electronegativity
Periodic trends electronegativity Ionization vs electronegativity
Ionization vs electronegativity Trend for ionization energy
Trend for ionization energy Alien periodic table periodic trends answers
Alien periodic table periodic trends answers Difference between modern periodic table and mendeleev
Difference between modern periodic table and mendeleev Hamlet act iii scene iii
Hamlet act iii scene iii Trend in ionization energy across a period
Trend in ionization energy across a period Order of increasing first ionization energy
Order of increasing first ionization energy What is calcium ionization energy
What is calcium ionization energy Ib chemistry atomic structure
Ib chemistry atomic structure Electronegativity trend exceptions
Electronegativity trend exceptions Order of increasing first ionization energy
Order of increasing first ionization energy Chapter 6 periodic table
Chapter 6 periodic table Do metals have high ionization energy
Do metals have high ionization energy Ionization energy coulomb's law
Ionization energy coulomb's law Ionization energy practice
Ionization energy practice Ionization energy snowman
Ionization energy snowman Why does atomic radius decrease across a period
Why does atomic radius decrease across a period First ionization energy of transition metals
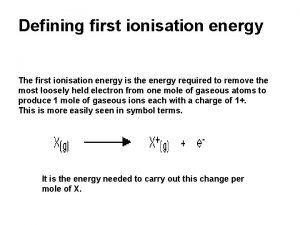
First ionization energy of transition metals Definition of first ionisation energy
Definition of first ionisation energy Ionization energy vs atomic number
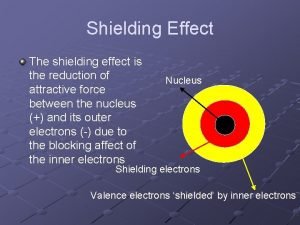
Ionization energy vs atomic number What is shielding effect
What is shielding effect