Atomic Radii IA IIIA Li Be B 1
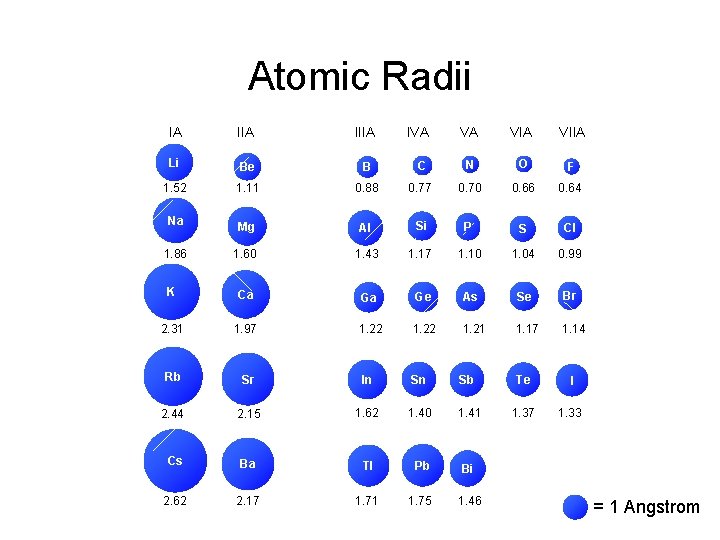
Atomic Radii IA IIIA Li Be B 1. 52 1. 11 Na 1. 86 IVA VA VIIA C N O F 0. 88 0. 77 0. 70 0. 66 0. 64 Mg Al Si P S Cl 1. 60 1. 43 1. 17 1. 10 1. 04 0. 99 K Ca Ga Ge As Se Br 2. 31 1. 97 1. 22 1. 21 1. 17 1. 14 Rb Sr In Sn Sb Te I 2. 44 2. 15 1. 62 1. 40 1. 41 1. 37 1. 33 Cs Ba Tl Pb Bi 2. 62 2. 17 1. 71 1. 75 1. 46 = 1 Angstrom
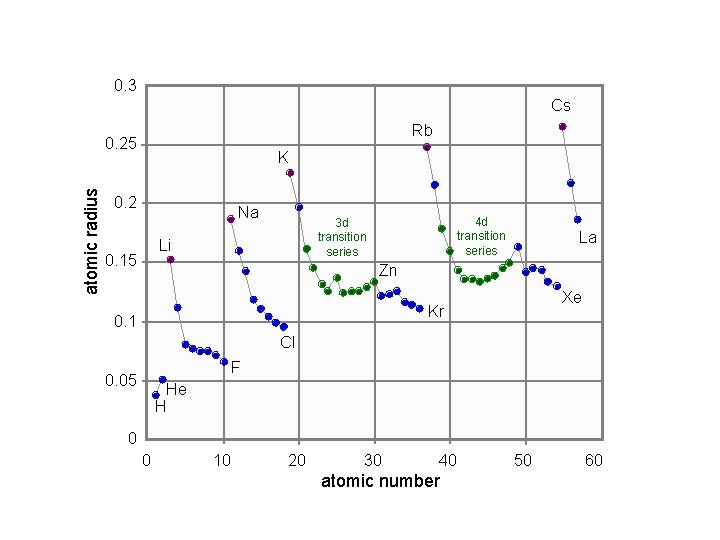
0. 3 Cs Rb atomic radius 0. 25 K 0. 2 Na Li 0. 15 4 d transition series 3 d transition series La Zn Xe Kr 0. 1 Cl F 0. 05 He H 0 0 10 20 30 40 atomic number 50 60
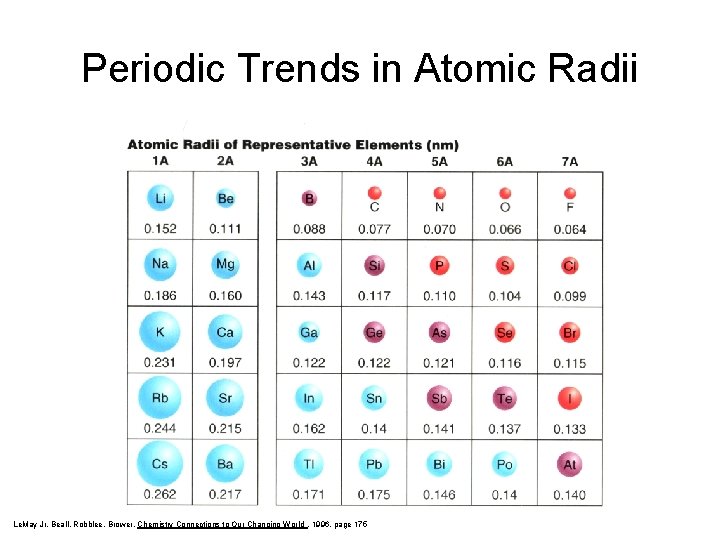
Periodic Trends in Atomic Radii Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 175
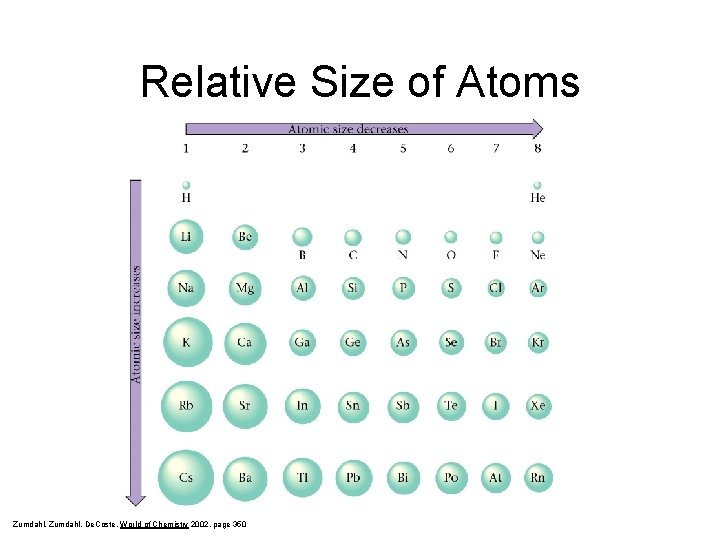
Relative Size of Atoms Zumdahl, De. Coste, World of Chemistry 2002, page 350
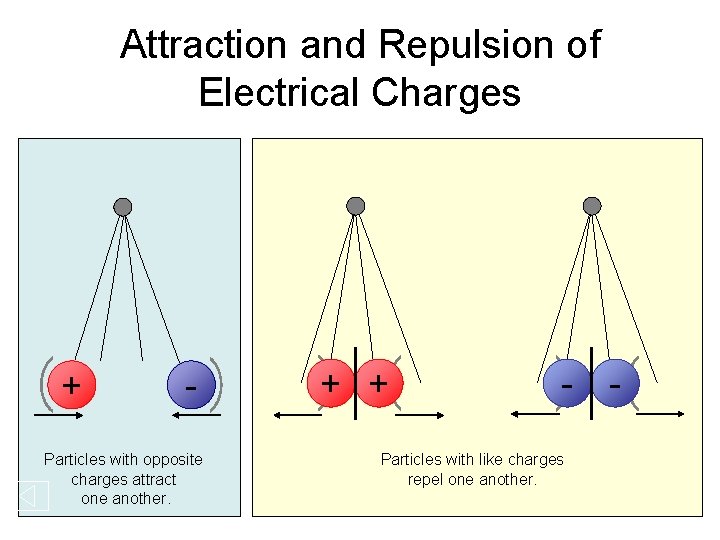
Attraction and Repulsion of Electrical Charges + - Particles with opposite charges attract one another. + + - Particles with like charges repel one another. -
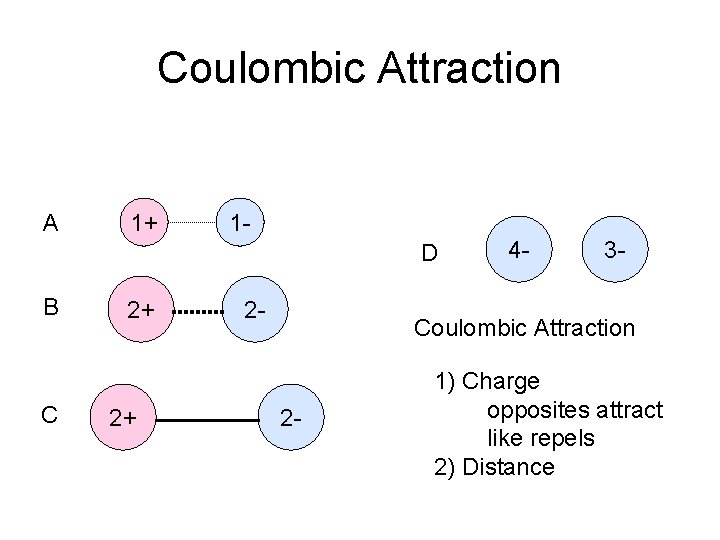
Coulombic Attraction A 1+ 1 D B C 2+ 2+ 2 - 4 - 3 - Coulombic Attraction 2 - 1) Charge opposites attract like repels 2) Distance
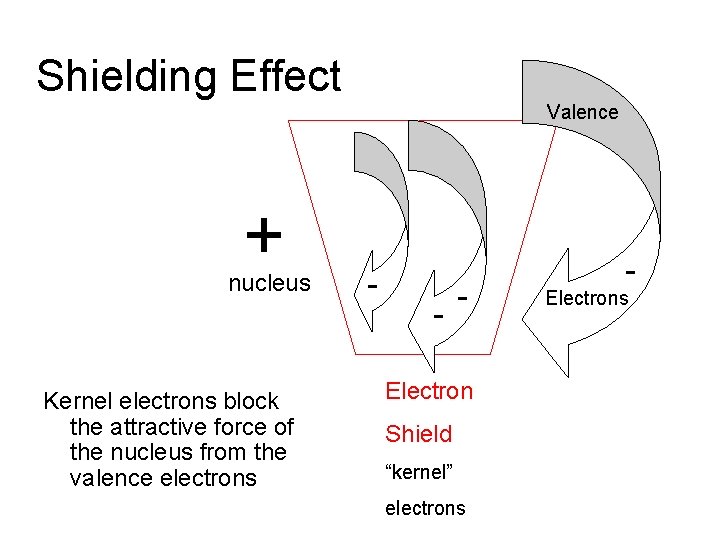
Shielding Effect Valence + nucleus Kernel electrons block the attractive force of the nucleus from the valence electrons - - - Electron Shield “kernel” electrons - Electrons
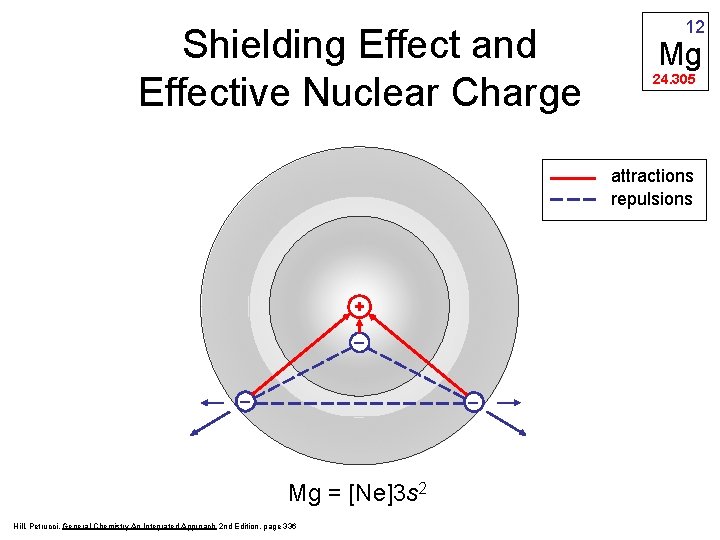
Shielding Effect and Effective Nuclear Charge 12 Mg 24. 305 attractions repulsions + _ _ _ Mg = [Ne]3 s 2 Hill, Petrucci, General Chemistry An Integrated Approach 2 nd Edition, page 336
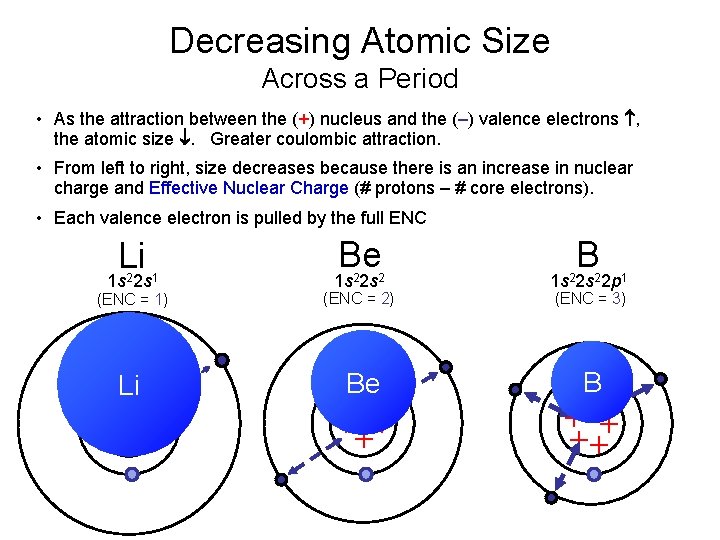
Decreasing Atomic Size Across a Period • As the attraction between the (+) nucleus and the (–) valence electrons , the atomic size . Greater coulombic attraction. • From left to right, size decreases because there is an increase in nuclear charge and Effective Nuclear Charge (# protons – # core electrons). • Each valence electron is pulled by the full ENC Li 1 s 22 s 1 Be B (ENC = 1) 1 s 22 s 2 (ENC = 2) 1 s 22 p 1 Li Be B ++ + + (ENC = 3) +++ ++
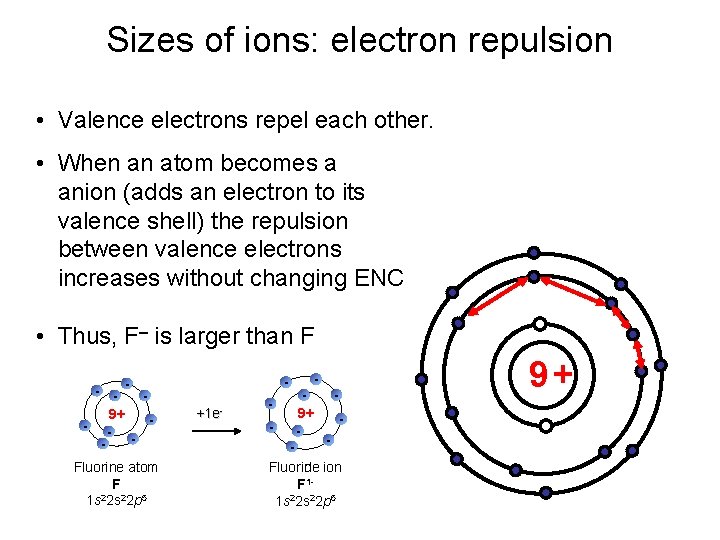
Sizes of ions: electron repulsion • Valence electrons repel each other. • When an atom becomes a anion (adds an electron to its valence shell) the repulsion between valence electrons increases without changing ENC • Thus, F– is larger than F - 9+ - Fluorine atom F 2 1 s 2 s 22 p 5 +1 e- - 9+ 9+ - - Fluorine ion Fluoride F 11 s 22 p 6
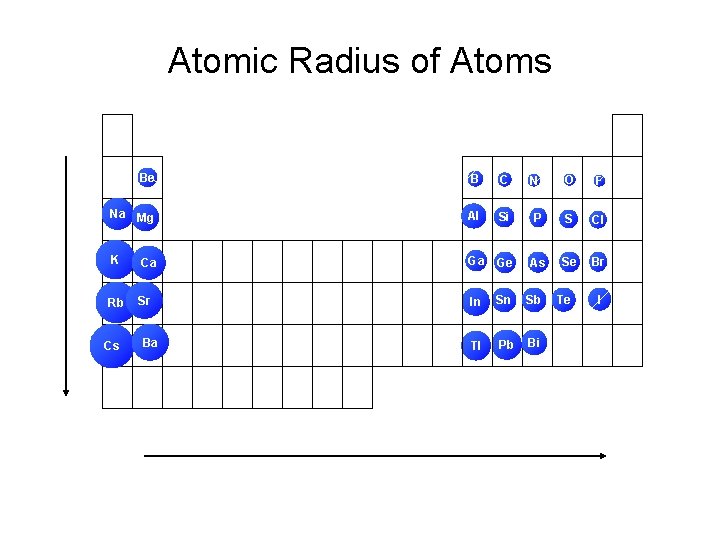
Atomic Radius of Atoms Be B C Na Mg Al Si K Ca Ga Ge Rb Sr In Sn Sb Tl Pb Bi Cs Ba O F P S Cl As Se Br N Te I
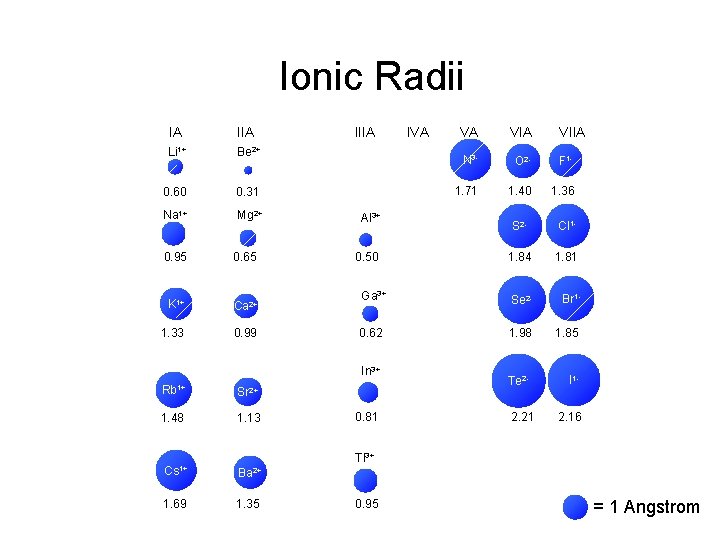
Atomic Ionic Radii IA IIIA IVA VA VIA Li 1+ Li Be 2+ Be 1. 52 0. 60 1+ Na Na VIIA B C NN 3 - OO 2 - F 1 F 1. 11 0. 31 0. 88 0. 77 0. 70 1. 71 0. 66 1. 40 0. 64 1. 36 Mg 2+ Mg Al 3+ Al Si P 2 SS 1 Cl. Cl 1. 43 0. 50 1. 17 1. 10 1. 04 1. 84 0. 99 1. 81 1. 86 0. 95 1. 60 0. 65 K K 1+ Ca Ca 2+ Ga 3+ Ge As Se 2 Se Br 1 Br 2. 31 1. 33 1. 97 0. 99 1. 22 0. 62 1. 21 1. 17 1. 98 1. 14 1. 85 Rb Rb 1+ Sr Sr 2+ In 3+ In Sn Sb 2. 44 1. 48 2. 15 1. 13 1. 62 0. 81 1. 40 1. 41 Tl 3+ Tl Pb Bi 1. 71 0. 95 1. 75 1. 46 Cs Cs 1+ Ba Ba 2+ 2. 62 1. 69 2. 17 1. 35 2 Te. Te 1. 37 2. 21 II 11. 33 2. 16 = 1 Angstrom
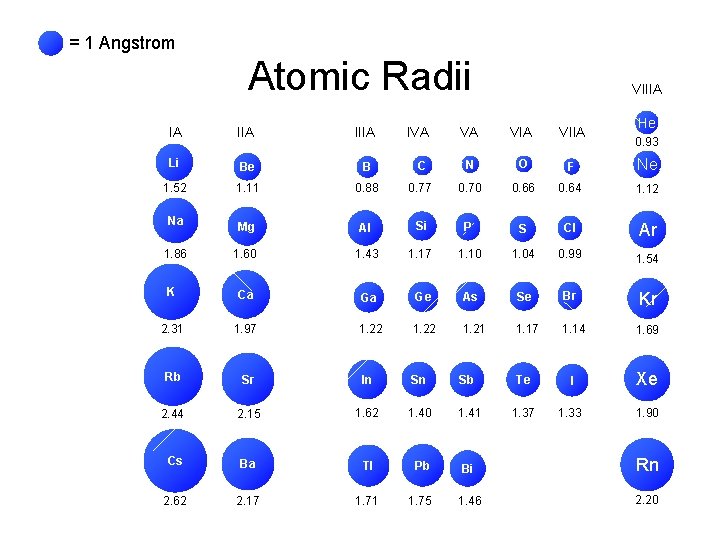
= 1 Angstrom Atomic Radii IA IIIA Li Be B 1. 52 1. 11 Na 1. 86 IVA VIIIA He VA VIIA C N O F Ne 0. 88 0. 77 0. 70 0. 66 0. 64 1. 12 Mg Al Si P S Cl Ar 1. 60 1. 43 1. 17 1. 10 1. 04 0. 99 1. 54 K Ca Ga Ge As Se Br Kr 2. 31 1. 97 1. 22 1. 21 1. 17 1. 14 1. 69 Rb Sr In Sn Sb Te I Xe 2. 44 2. 15 1. 62 1. 40 1. 41 1. 37 1. 33 1. 90 Cs Ba Tl Pb Bi Rn 2. 62 2. 17 1. 71 1. 75 1. 46 2. 20 0. 93
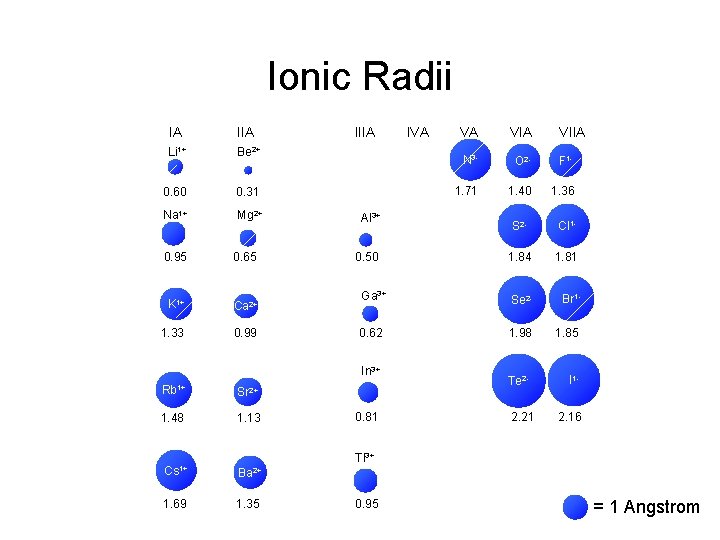
Ionic Radii IA IIA Li 1+ Be 2+ 0. 60 0. 31 Na 1+ Mg 2+ 0. 95 0. 65 K 1+ Ca 2+ 1. 33 0. 99 IIIA Al 3+ Sr 2+ 1. 48 1. 13 VA VIIA N 3 - O 2 - F 1 - 1. 71 1. 40 1. 36 S 2 - Cl 1 - 1. 84 1. 81 Ga 3+ Se 2 - Br 1 - 0. 62 1. 98 1. 85 Te 2 - I 1 - 0. 50 In 3+ Rb 1+ IVA 0. 81 2. 21 2. 16 Tl 3+ Cs 1+ Ba 2+ 1. 69 1. 35 0. 95 = 1 Angstrom
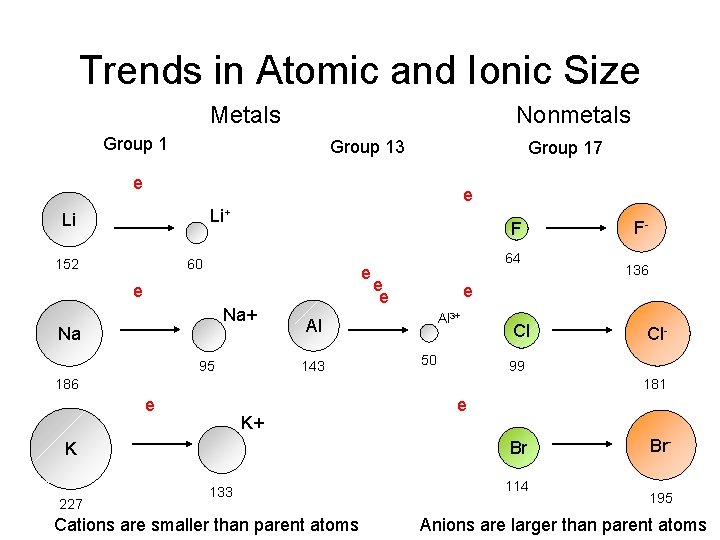
Trends in Atomic and Ionic Size Metals Nonmetals Group 13 Group 17 e e Li+ Li 152 F 60 e e Na+ Na 95 64 e e Al 3+ 50 Cl Cl- 99 186 181 e K+ e Br K 227 136 e Al 143 F- 133 Cations are smaller than parent atoms 114 Br 195 Anions are larger than parent atoms
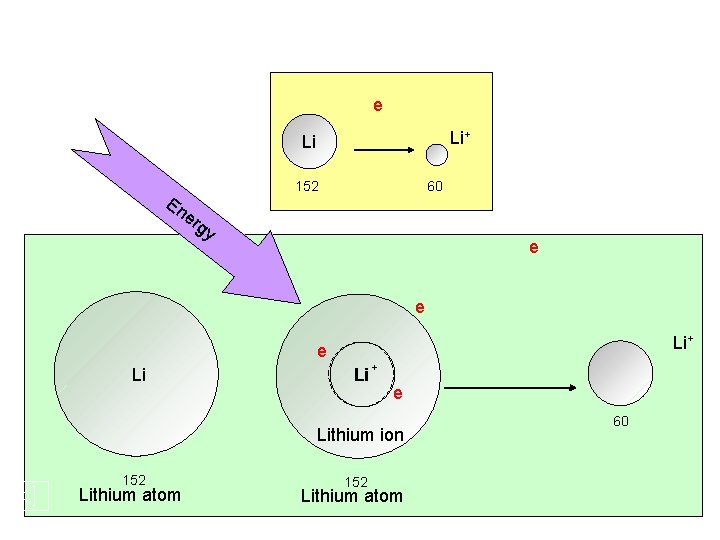
e Li+ Li En er 152 60 gy e e Li+ e Li Li + e Lithium ion 152 Lithium atom 60
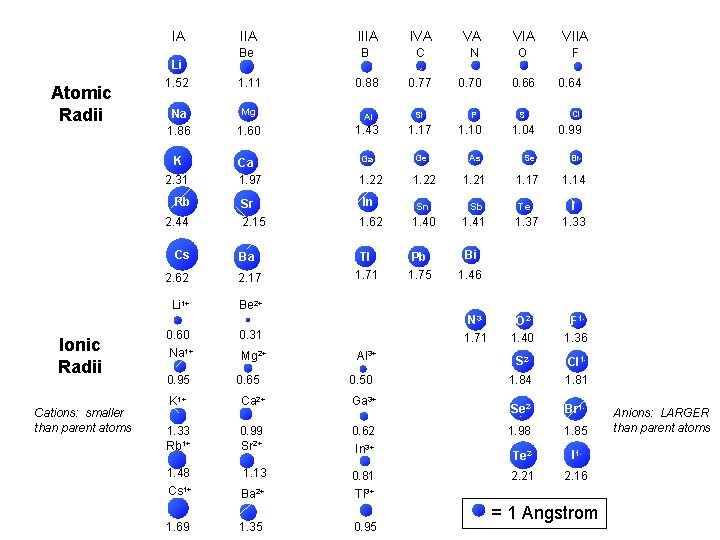
IA Atomic Radii Li 1. 52 Na 1. 86 IVA VA VIIA Be B C N O F 1. 11 0. 88 0. 77 Mg Al Si 1. 60 1. 43 1. 17 Ca 1. 97 Ga Ge 1. 22 Sr 0. 70 P 0. 66 S 0. 64 Cl 1. 04 0. 99 As Se Br 1. 22 1. 21 1. 17 1. 14 In Sn Sb Te 2. 15 1. 62 1. 40 1. 41 1. 37 I 1. 33 Ba Tl Pb Bi 2. 62 2. 17 1. 71 1. 75 1. 46 Li 1+ Be 2+ 2. 31 Rb 2. 44 Cs Cations: smaller than parent atoms IIIA 1. 10 K Ionic Radii IIA 0. 60 Na 1+ 0. 31 0. 95 0. 65 Mg 2+ N 31. 71 Al 3+ 0. 50 K 1+ Ca 2+ Ga 3+ 1. 33 Rb 1+ 0. 99 Sr 2+ 0. 62 1. 48 Cs 1+ 1. 13 0. 81 Ba 2+ Tl 3+ 1. 69 1. 35 0. 95 In 3+ O 21. 40 F 11. 36 S 21. 84 Cl 11. 81 Se 2 - Br 1 - 1. 98 1. 85 Te 2 - I 1 - 2. 21 2. 16 = 1 Angstrom Anions: LARGER than parent atoms
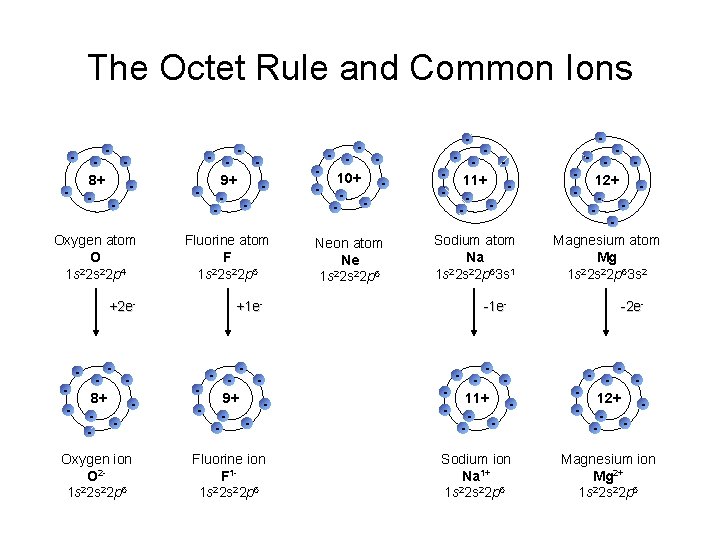
The Octet Rule and Common Ions - - 8+ - - Oxygen atom O 2 1 s 2 s 22 p 4 - - 9+ - - 10+ - - 11+ - - Fluorine atom F 2 1 s 2 s 22 p 5 Neon atom Ne 2 1 s 2 s 22 p 6 Sodium atom Na 2 1 s 2 s 22 p 63 s 1 - - 12+ - Magnesium atom Mg 2 1 s 2 s 22 p 63 s 2 +2 e- +1 e- 8+ - - 9+ - - 11+ - - 12+ - - Oxygen ion O 21 s 22 p 6 Fluorine ion F 11 s 22 p 6 Sodium ion Na 1+ 1 s 22 p 6 Magnesium ion Mg 2+ 1 s 22 p 6 - -1 e- - -2 e-
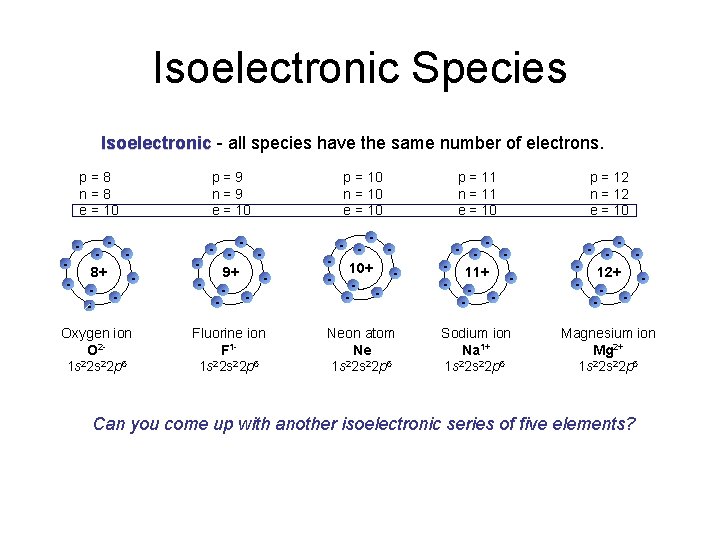
Isoelectronic Species Isoelectronic - all species have the same number of electrons. p=8 n=8 e = 10 p=9 n=9 e = 10 p = 10 n = 10 e = 10 p = 11 n = 11 e = 10 p = 12 n = 12 e = 10 8+ - - 9+ - - 10+ - - 11+ - - 12+ - - Oxygen ion O 21 s 22 p 6 Fluorine ion F 11 s 22 p 6 Neon atom Ne 2 1 s 2 s 22 p 6 Sodium ion Na 1+ 1 s 22 p 6 Magnesium ion Mg 2+ 1 s 22 p 6 - - Can you come up with another isoelectronic series of five elements?
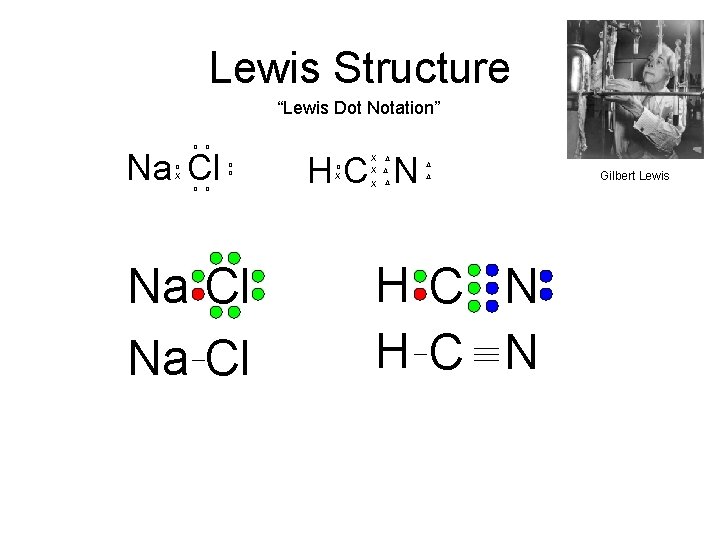
Lewis Structure “Lewis Dot Notation” o o Na Cl o X o o Na Cl HC N o X X D X D D D H C N Gilbert Lewis
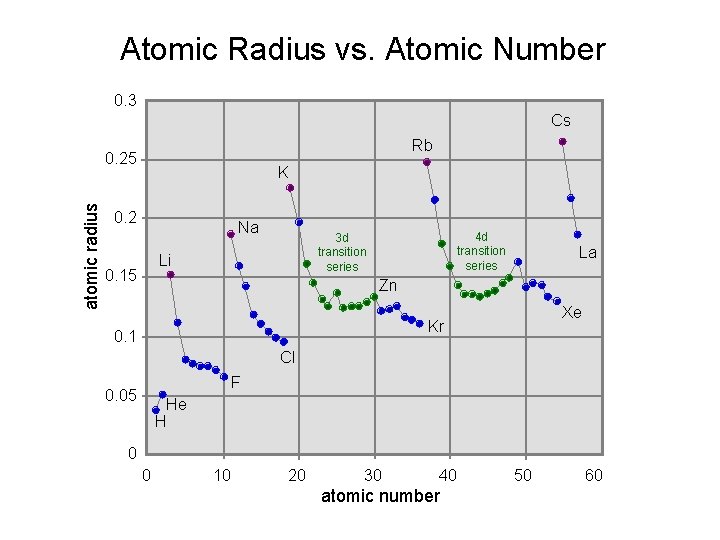
Atomic Radius vs. Atomic Number 0. 3 Cs Rb atomic radius 0. 25 K 0. 2 Na Li 0. 15 4 d transition series 3 d transition series La Zn Xe Kr 0. 1 Cl F 0. 05 He H 0 0 10 20 30 40 atomic number 50 60
- Slides: 21