Trends in Atomic Radius Ionization Energy and Electronegativity



















- Slides: 28

Trends in Atomic Radius, Ionization Energy and Electronegativity

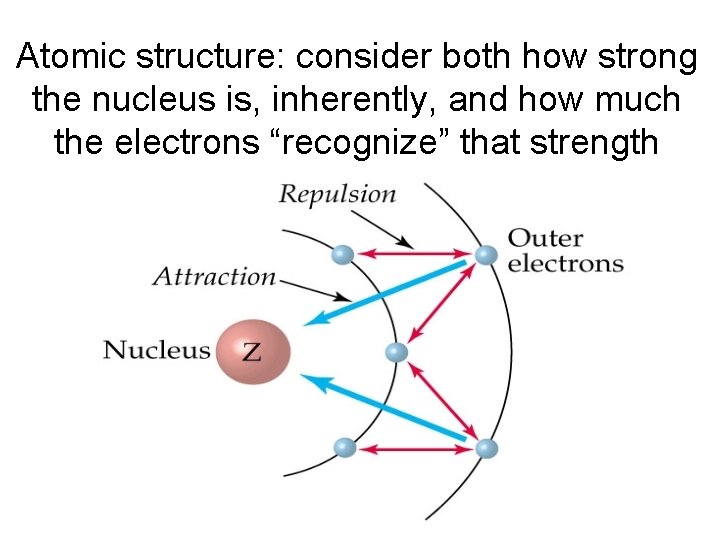
Trends in Atomic Radius, Ionization Energy and Electronegativity can be explained by the atom’s structure • Strength of the Nucleus (As the number of protons in the nucleus increases, the nucleus is more attractive to electrons. ) • Shielding means “blocking”. If electrons are shielded or “blocked” they won’t feel the attractive pull of the nucleus.

Atomic structure: consider both how strong the nucleus is, inherently, and how much the electrons “recognize” that strength

Analogy A street performer is like a nucleus. It could be attractive to its audience, but one must consider the following: • How talented is the performer—how strongly does he or she attract onlookers? • How much are the onlookers blocked from being able to see the performer?

By considering both the strength of the nucleus and the amount of shielding electrons experience, we can describe and explain trends in atomic radius, ionization energy and electronegativity.

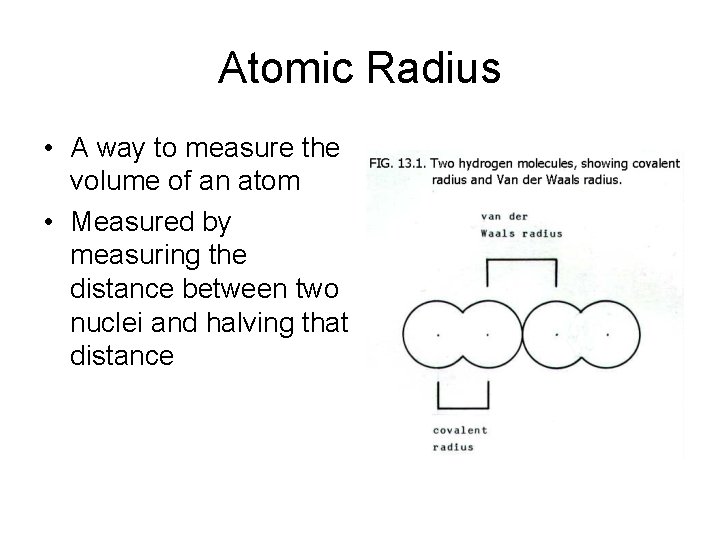
Atomic Radius • A way to measure the volume of an atom • Measured by measuring the distance between two nuclei and halving that distance

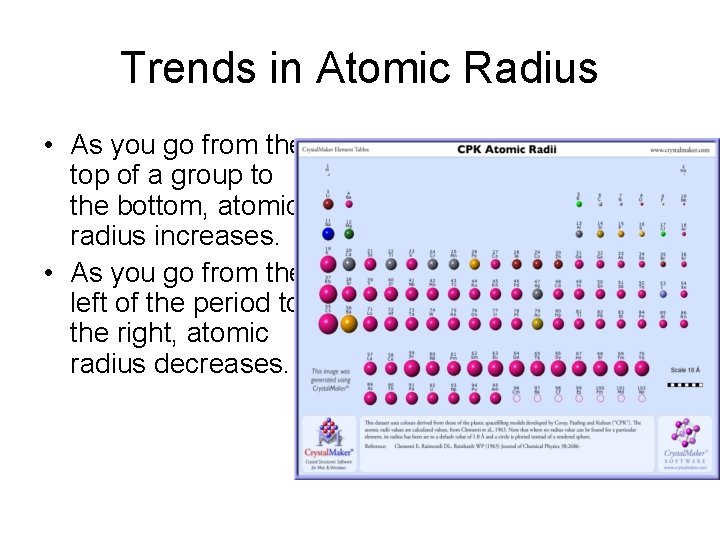
Trends in Atomic Radius • As you go from the top of a group to the bottom, atomic radius increases. • As you go from the left of the period to the right, atomic radius decreases.

Atomic Radius Trends Explained • Group Trends From the top of a group to the bottom of a group, the number of protons increases, but so does the number of energy levels. Nuclear charge increases, but so does shielding. Electrons in the outer energy levels are more shielded, and they don’t experience the full attractiveness of the nucleus and they tend to “stray” further, making the radius larger.

Note how the outside audience members are shielded

Trends in Atomic Radius Continued… • Period Trends As you go from left to right across the period, the nuclear charge increases, but the shielding does not. The nucleus gets stronger, and all electrons in the period feel the same “draw” toward the nucleus.

Would the distracted audience member be more attentive if the act were better?

We can explain trends in ionization energy and electronegativity in the same way.

Ionization Energy • The amount of energy required to remove an electron • A high ionization energy means a lot of energy is required to remove an electron • First ionization energy = the amount of energy required to remove the first electron • Second ionization energy = the amount of energy required to remove the second electron (after the first has already been removed)

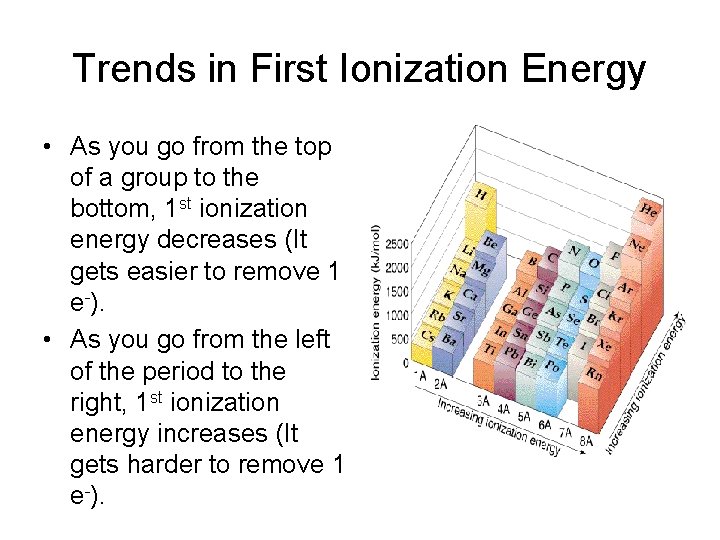
Trends in First Ionization Energy • As you go from the top of a group to the bottom, 1 st ionization energy decreases (It gets easier to remove 1 e-). • As you go from the left of the period to the right, 1 st ionization energy increases (It gets harder to remove 1 e-).

Trends in Ionization Energy Explained Again it is all about the atomic structure: Nuclear Charge and Shielding! When we consider the atom, we must ask, “how strong is its nucleus? ” “How much are the electrons we want to remove shielded? ”

Trends in Ionization Energy Explained • Group Trends From the top of a group to the bottom of a group, the number of protons increases, but so does the number of energy levels. Nuclear charge increases, but so does shielding. Electrons in the outer energy levels are more shielded, and they don’t experience the full attractiveness of the nucleus so it is easier to pull them away from the nucleus.

Explanation of Trends in Ionization Energy Continued… • Period Trends As you go from left to right across the period, the nuclear charge increases, but the shielding does not. The nucleus gets stronger and electrons are held more tightly, making them harder to remove.

Comparing first, second and third ionization energies Not only is it useful to analyze trends in first ionization energy, but we can use differences in first, second and third ionization energies to predict the kind of ion an ion will form

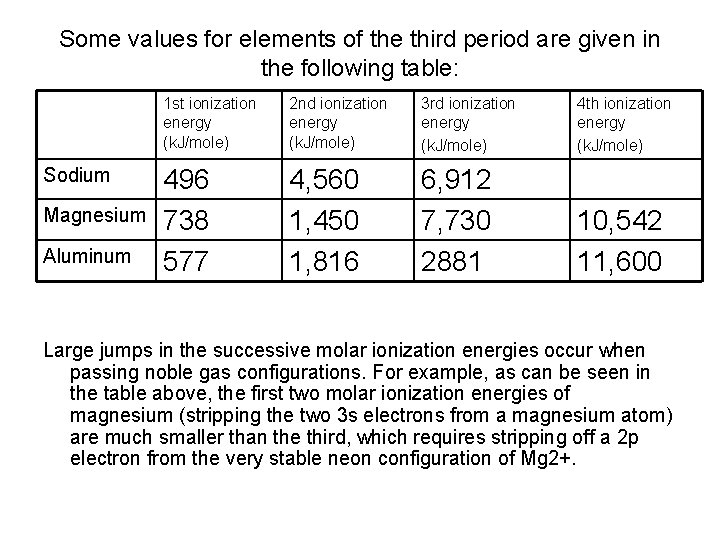
Some values for elements of the third period are given in the following table: Sodium Magnesium Aluminum 1 st ionization energy (k. J/mole) 2 nd ionization energy (k. J/mole) 3 rd ionization energy (k. J/mole) 4 th ionization energy (k. J/mole) 496 738 577 4, 560 1, 450 1, 816 6, 912 7, 730 2881 10, 542 11, 600 Large jumps in the successive molar ionization energies occur when passing noble gas configurations. For example, as can be seen in the table above, the first two molar ionization energies of magnesium (stripping the two 3 s electrons from a magnesium atom) are much smaller than the third, which requires stripping off a 2 p electron from the very stable neon configuration of Mg 2+.

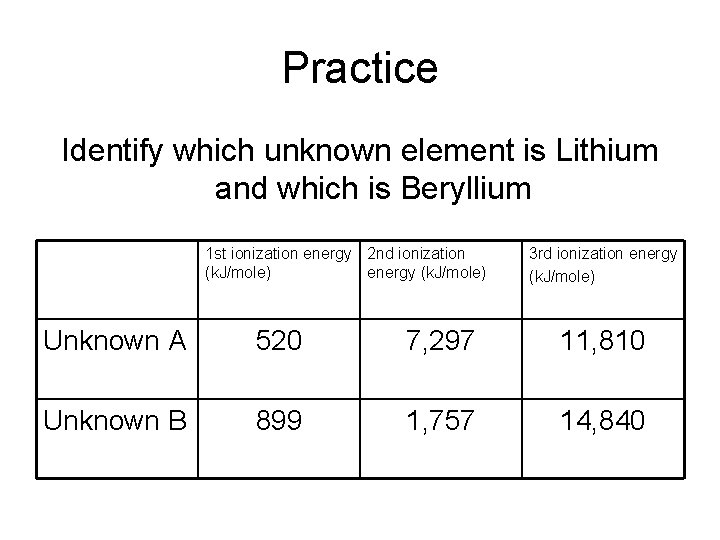
Practice Identify which unknown element is Lithium and which is Beryllium 1 st ionization energy 2 nd ionization (k. J/mole) energy (k. J/mole) 3 rd ionization energy (k. J/mole) Unknown A 520 7, 297 11, 810 Unknown B 899 1, 757 14, 840

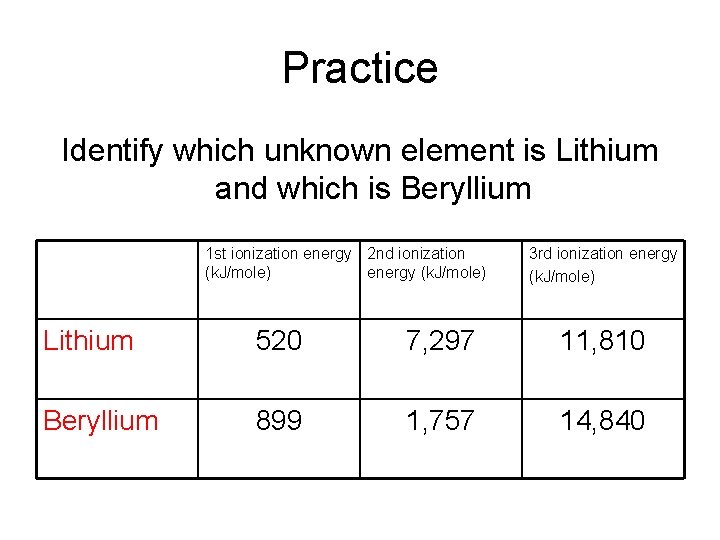
Practice Identify which unknown element is Lithium and which is Beryllium 1 st ionization energy 2 nd ionization (k. J/mole) energy (k. J/mole) 3 rd ionization energy (k. J/mole) Lithium 520 7, 297 11, 810 Beryllium 899 1, 757 14, 840

Electronegativity • The ability of an atom to attract electrons to itself • Only measured when the atom is combined in a compound • Linus Pauling was the first to define and measure electronegativity. The units are named after him and range from 0. 7 -4. 0 Paulings

Trends in Electronegativity • As you go from the top of a group to the bottom, electronegativity decreases. • As you go from the left of the period to the right, electronegativity increases.

Trends in Ionization Energy Explained Again it is all about the atomic structure: Nuclear Charge and Shielding! When we consider the atom, we must ask, “how strong is its nucleus? ” “How much are the electrons shielded from being attracted to the full pull of the nucleus? ”

Trends in Electronegativity Explained • Group Trends From the top of a group to the bottom of a group, the number of protons increases, but so does the number of energy levels. Nuclear charge increases, but so does shielding. Electrons in the outer energy levels are more shielded, and they aren’t as attracted to the nucleus.

Explanation of Trends in Electronegativity Continued… • Period Trends As you go from left to right across the period, the nuclear charge increases, but the shielding does not. The nucleus gets stronger and it is better able to attract electrons • Note: electronegativity of the Noble gases can not be measured.
Based on these trends… 1) What do you expect to be the most reactive metal? 2) What do you expect to be the most reactive nonmetal? (Hint: What do metals do to react? How do you measure that? What is the trend in that property? Hint what do nonmetals do to react? How do you measure that? What is the trend in that property? )

1) What do you expect to be the most reactive metal? Discounting the radioactive metals, like francium, the most reactive metal is cesium. 2) What do you expect to be the most reactive nonmetal? Fluorine