Periodic trends review game Periodic Trends Review Game




















































- Slides: 53

Periodic trends review game Periodic Trends Review Game Are you ready to play?

Periodic trends review game Periodic Trends Review Game Question #1: What are the members of Group 17 (7 A) called?

Periodic trends review game Periodic Trends Review Game Answer #1: Group 17 members are called halogens.

Periodic trends review game Periodic Trends Review Game Question #2: What is an element called that has properties of both metals and non-metals?

Periodic trends review game Periodic Trends Review Game Answer #2: Metalloids have properties of both metals and non-metals.

Periodic trends review game Periodic Trends Review Game Question #3: Do non-metals have a relatively high or low electronegativity?

Periodic trends review game Periodic Trends Review Game Answer #3: Non-metals have a relatively high electronegativity.

Periodic trends review game Periodic Trends Review Game Question #4: Name 2 properties of metals.

Periodic trends review game Periodic Trends Review Game Answer #4: (2 needed) Metals have a luster; they are ductile, malleable, and conductors of heat and electricity.

Periodic trends review game Periodic Trends Review Game Question #5: What is the term that describes the ability of a bonded atom to draw an electron to itself?

Periodic trends review game Periodic Trends Review Game Answer #5: Electronegativity refers to the ability of a bonded atom to draw electrons to itself.

Periodic trends review game Periodic Trends Review Game Question #6: What are the members of Group 2(2 A) called?

Periodic trends review game Periodic Trends Review Game Answer #6: The members of Group 2(2 A) are called alkaline earth metals.

Periodic trends review game Periodic Trends Review Game Question #7: Who rearranged the periodic table by atomic number?

Periodic trends review game Periodic Trends Review Game Answer #7: Moseley rearranged the periodic table by atomic number.

Periodic trends review game Periodic Trends Review Game Question #8: How does the size of a Clion compare to the neutral Cl atom?

Periodic trends review game Periodic Trends Review Game Answer #8: The Cl- ion is larger than the neutral Cl atom.

Periodic trends review game Periodic Trends Review Game Question #9: Arrange the particles from smallest to largest radius: K, Na, K+, Na+

Periodic trends review game Periodic Trends Review Game Answer #9: From smallest to largest radius the particles are: Na+, Na, K+, K

Periodic trends review game Periodic Trends Review Game Question #10: An element is a highly reactive, soft metal located in group 1. Which element could it be? Li, Al, C or Mg

Periodic trends review game Periodic Trends Review Game Answer #10: The element would most likely be Li.

Periodic trends review game Periodic Trends Review Game Question #11: Who developed the first widely used periodic table?

Periodic trends review game Periodic Trends Review Game Answer #11: Mendeleev

Periodic trends review game Periodic Trends Review Game Question #12: Which element is most likely to be a poor conductor? S, Si, Ag, or Sr

Periodic trends review game Periodic Trends Review Game Answer #12: S, a nonmetal, is most likely to be a poor conductor.

Periodic trends review game Periodic Trends Review Game Question #13: Which group is called the noble gases?

Periodic trends review game Periodic Trends Review Game Answer #13: The noble gases are in group 18(8 A).

Periodic trends review game Periodic Trends Review Game Question #14: What are the elements in groups 3 -12 called?

Periodic trends review game Periodic Trends Review Game Answer #14: They are called transition metals.

Periodic trends review game Periodic Trends Review Game Question #15: Which of the following is not a representative element? A) Li B) C C) He D) U

Periodic trends review game Periodic Trends Review Game Answer #15: Choice D) U (Uranium) is not a representative element.

Periodic trends review game Periodic Trends Review Game Question #16: Place the following elements in order of increasing electronegativity. Cl, I, Br, F

Periodic trends review game Periodic Trends Review Game Answer #16: I, Br, Cl, F represents the correct order for increasing electronegativity

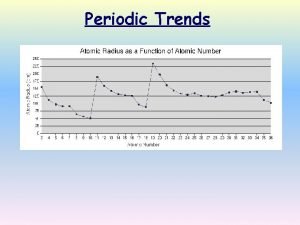
Periodic trends review game Periodic Trends Review Game Question #17: Place the following elements in order of increasing atomic radius - C, O, F, N

Periodic trends review game Periodic Trends Review Game Answer #17: F, O, N, C represents the correct order for increasing atomic radius

Periodic trends review game Periodic Trends Review Game Question #18: What element is found in Group 13 and in Period 2?

Periodic trends review game Periodic Trends Review Game Answer #18: Boron is found in Group 13 and Period 2.

Periodic trends review game Periodic Trends Review Game Question #19: How many valence electrons do alkaline earth metals have?

Periodic trends review game Periodic Trends Review Game Answer #19: Alkaline earth metals (Group 2/2 A) have 2 valence electrons.

Periodic trends review game Periodic Trends Review Game Question #20: Which of the following elements is located in the f-block of the periodic table? A) Beryllium B) Titanium C) Plutonium D) Platinum

Periodic trends review game Periodic Trends Review Game Answer #20: Choice C – Plutonium (Pu) is located in the f-block of the periodic table.

Periodic trends review game Periodic Trends Review Game Question #21: How many valence electrons does iodine have?

Periodic trends review game Periodic Trends Review Game Answer #21: Iodine has 7 valence electrons.

Periodic trends review game Periodic Trends Review Game Question #22: Which element is a halogen? Oxygen, Beryllium, Chlorine or Neon?

Periodic trends review game Periodic Trends Review Game Answer #22: Chlorine is a halogen.

Periodic trends review game Periodic Trends Review Game Question #23: Which element has the lowest ionization energy? F, Ca, Rb, or Na

Periodic trends review game Periodic Trends Review Game Answer #23: Rb has the lowest ionization energy.

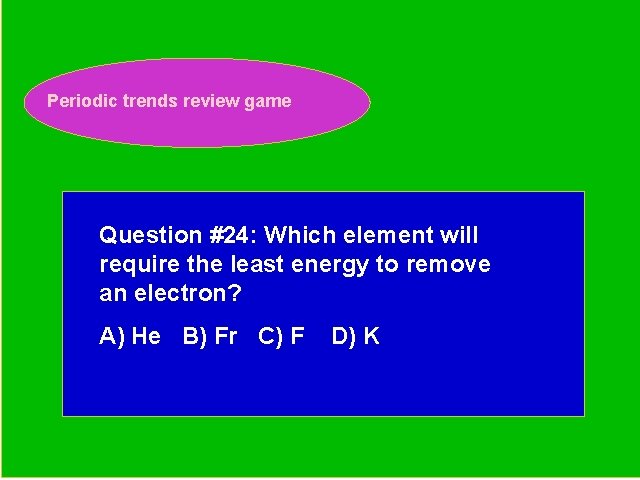
Periodic trends review game Periodic Trends Review Game Question #24: Which element will require the least energy to remove an electron? A) He B) Fr C) F D) K

Periodic trends review game Periodic Trends Review Game Answer # 24: Choice B- Fr requires the least amount of energy to remove an electron; it has the lowest ionization energy.

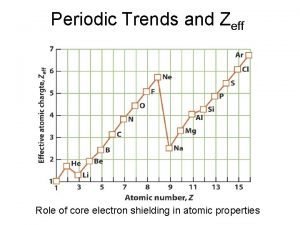
Periodic trends review game Periodic Trends Review Game Question 25: Define shielding.

Periodic trends review game Periodic Trends Review Game Answer # 25: Shielding describes the number of electron layers between the nucleus of an atom and the outermost level of electrons.

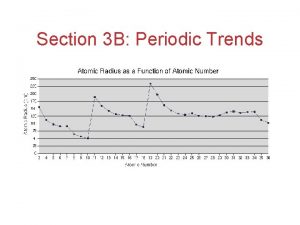
Periodic trends review game Periodic Trends Review Game Question 26: What properties of atoms are responsible for the trends we observe in atomic radius, ionization energy, and electronegativity?

Periodic trends review game Periodic Trends Review Game Answer # 26: The properties of the atom that are responsible for the trends we observe in atomic radius, ionization energy, and electronegativity are shielding and effective nuclear charge.
 Periodic trend
Periodic trend 15/999 mass street periodic table, o 8
15/999 mass street periodic table, o 8 Periodic trends game
Periodic trends game Periodic table with properties
Periodic table with properties Ionic radius periodic trend
Ionic radius periodic trend Cheat periodic table
Cheat periodic table Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Graphing periodic trends
Graphing periodic trends Oxidation trends periodic table
Oxidation trends periodic table Periodic trend definition
Periodic trend definition Periodic trend for zeff
Periodic trend for zeff Periodic trends activity worksheet
Periodic trends activity worksheet Periodic trends practice quiz
Periodic trends practice quiz Periodic trends in reactivity
Periodic trends in reactivity Summary of periodic trends
Summary of periodic trends Ionization energy practice problems
Ionization energy practice problems Coulomb's law electronegativity
Coulomb's law electronegativity Electron affinity trends
Electron affinity trends Increasing atomic size
Increasing atomic size نصف القطر الذري
نصف القطر الذري Periodic trends definition
Periodic trends definition Periodic trends acidity
Periodic trends acidity Periodic table trends
Periodic table trends Periodic trends
Periodic trends Periodic trends in elemental properties
Periodic trends in elemental properties Patterns in the periodic table
Patterns in the periodic table Atomic radius ionization energy
Atomic radius ionization energy Periodic trends in properties of elements
Periodic trends in properties of elements Periodic trends in elemental properties
Periodic trends in elemental properties Periodic trends in elemental properties
Periodic trends in elemental properties Atomic radius definition
Atomic radius definition Density trend periodic table
Density trend periodic table Chemical bonding assignment
Chemical bonding assignment Periodic trends
Periodic trends Do metals have high ionization energy
Do metals have high ionization energy Chapter 6 periodic table
Chapter 6 periodic table The periodic table and periodic law chapter 6
The periodic table and periodic law chapter 6 Section 1 guided reading and review labor market trends
Section 1 guided reading and review labor market trends Periodic table review
Periodic table review Chapter 14 review activity the periodic table
Chapter 14 review activity the periodic table Universal periodic review kenya
Universal periodic review kenya Periodic service review
Periodic service review Game theory pirate game
Game theory pirate game Farming game rules
Farming game rules A formal approach to game design and game research
A formal approach to game design and game research Game lab game theory
Game lab game theory Liar game game theory
Liar game game theory Liar game game theory
Liar game game theory Trash ball review game
Trash ball review game Climax of romeo and juliet
Climax of romeo and juliet Genetics jeopardy review game
Genetics jeopardy review game Civil war jeopardy
Civil war jeopardy Evolution jeopardy review game
Evolution jeopardy review game The reason the hypodermis acts as a shock absorber is that
The reason the hypodermis acts as a shock absorber is that