Periodic Table Basics Your cheat sheet On the

Periodic Table Basics “Your cheat sheet”
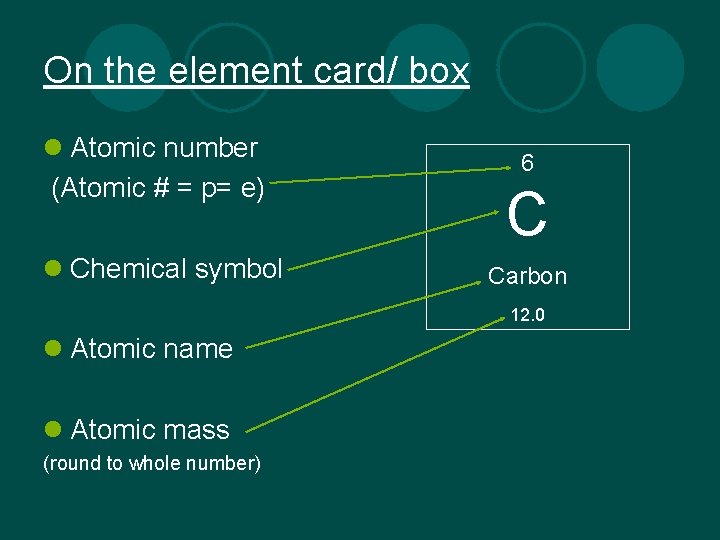
On the element card/ box l Atomic number (Atomic # = p= e) l Chemical symbol 6 C Carbon 12. 0 l Atomic name l Atomic mass (round to whole number)
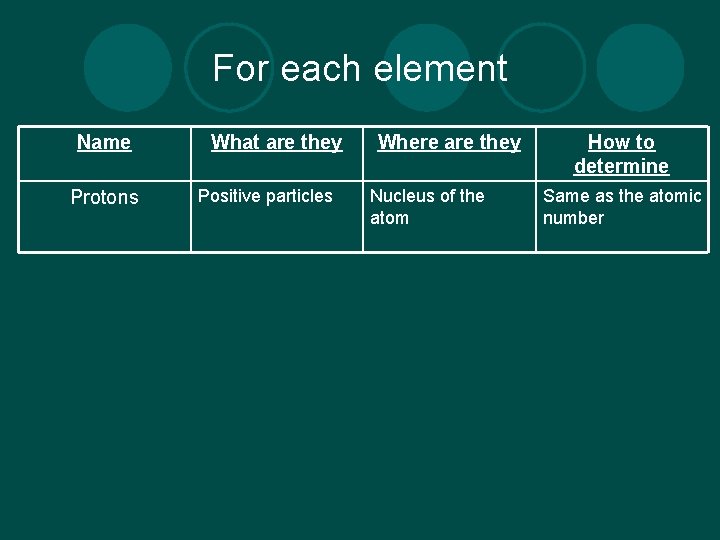
For each element Name Protons What are they Positive particles Where are they Nucleus of the atom How to determine Same as the atomic number
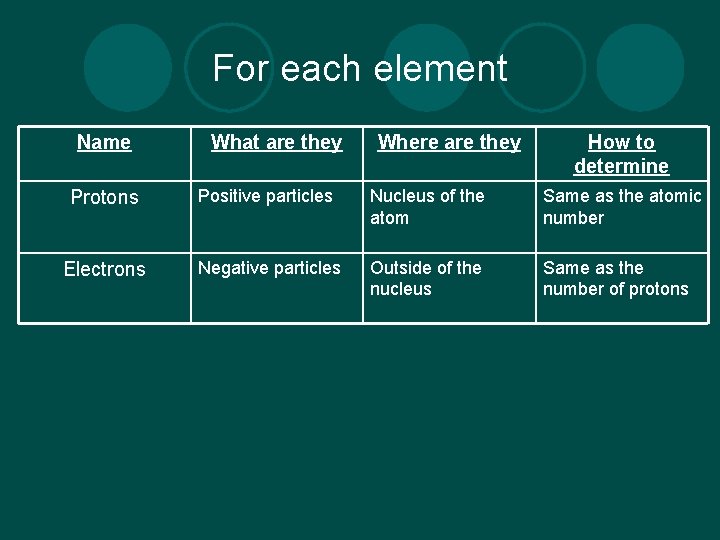
For each element Name What are they Where are they How to determine Protons Positive particles Nucleus of the atom Same as the atomic number Electrons Negative particles Outside of the nucleus Same as the number of protons
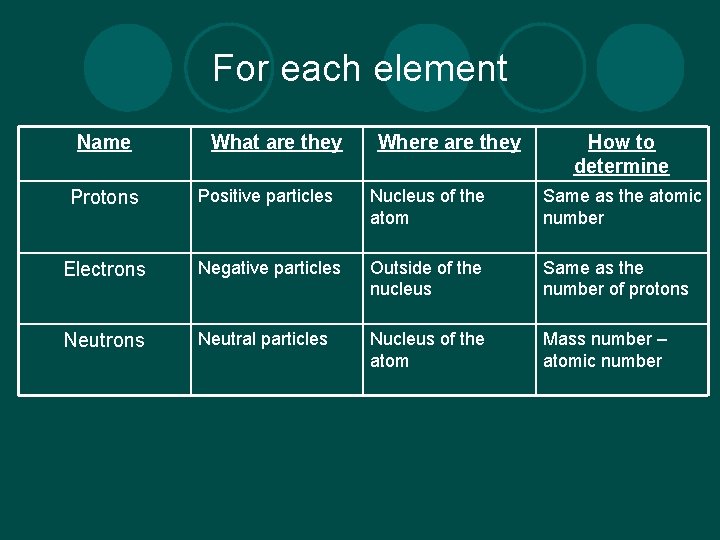
For each element Name What are they Where are they How to determine Protons Positive particles Nucleus of the atom Same as the atomic number Electrons Negative particles Outside of the nucleus Same as the number of protons Neutral particles Nucleus of the atom Mass number – atomic number
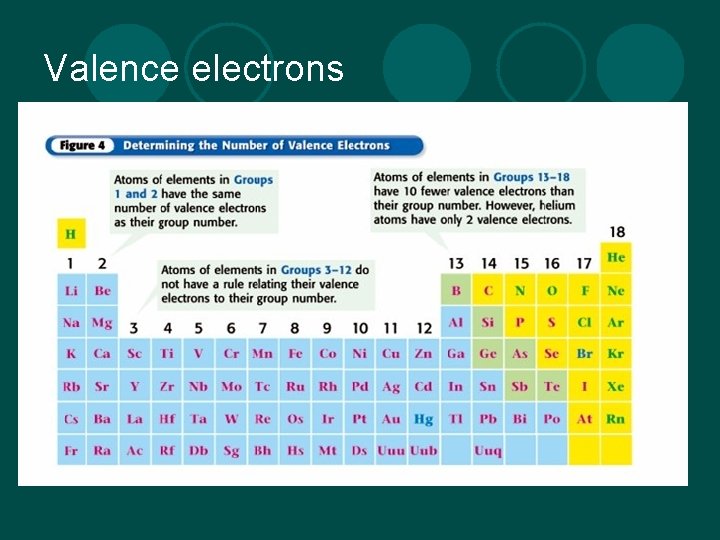
Valence electrons
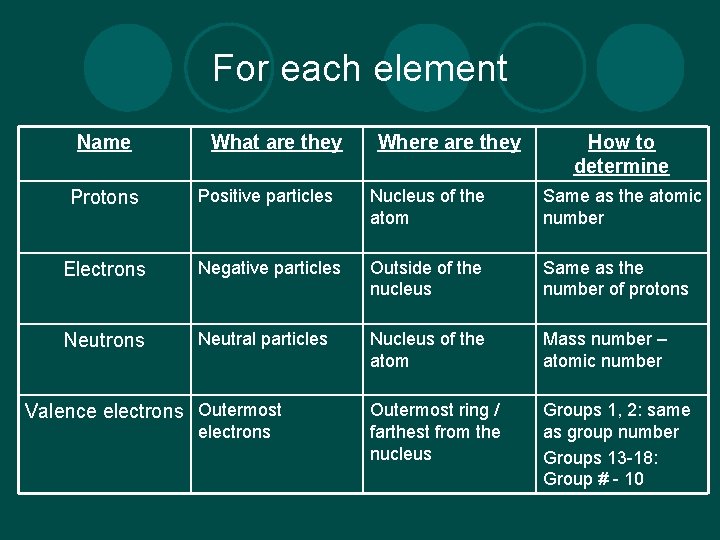
For each element Name What are they Where are they How to determine Protons Positive particles Nucleus of the atom Same as the atomic number Electrons Negative particles Outside of the nucleus Same as the number of protons Neutral particles Nucleus of the atom Mass number – atomic number Outermost ring / farthest from the nucleus Groups 1, 2: same as group number Groups 13 -18: Group # - 10 Valence electrons Outermost electrons
- Slides: 7