COLLIGATIVE PROPERTIES of SOLUTIONS Why do we need







- Slides: 21

COLLIGATIVE PROPERTIES of SOLUTIONS

Why do we need to add salt to ice to make ice cream? https: //www. youtube. com/watch? v=Fmavy. OIQIz 0 Ice and Salt

The salt added to the ice lowers the freezing point of the ice so that the temperature surrounding the ice cream is even colder than freezing. The lowering of the freezing point of water is a colligative property. A colligative property is a property of solutions that depends only upon the number of solute particles, not upon their identity. Three important colligative properties of solutions are: • Freezing-point depression • Vapor-pressure lowering • Boiling-point elevation

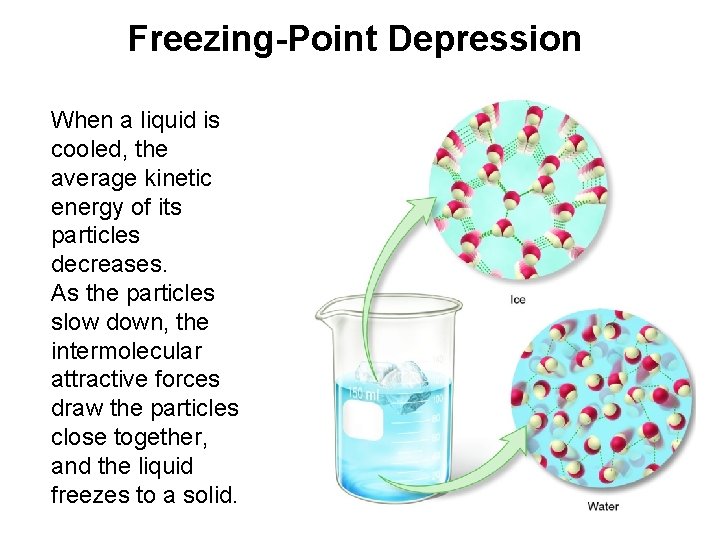
Freezing-Point Depression When a liquid is cooled, the average kinetic energy of its particles decreases. As the particles slow down, the intermolecular attractive forces draw the particles close together, and the liquid freezes to a solid.

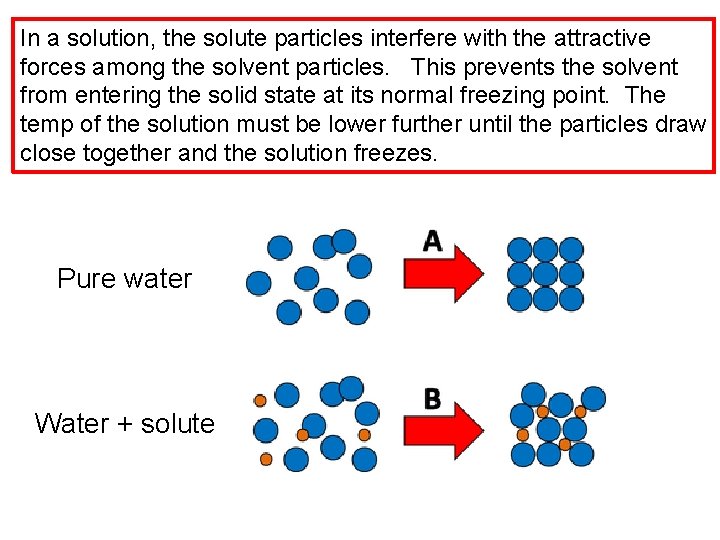
In a solution, the solute particles interfere with the attractive forces among the solvent particles. This prevents the solvent from entering the solid state at its normal freezing point. The temp of the solution must be lower further until the particles draw close together and the solution freezes. Pure water Water + solute

The freezing point of a solution is lower than that of a pure solvent. Salt lowers the freezing point of water. Antifreeze lowers the freezing point of water.

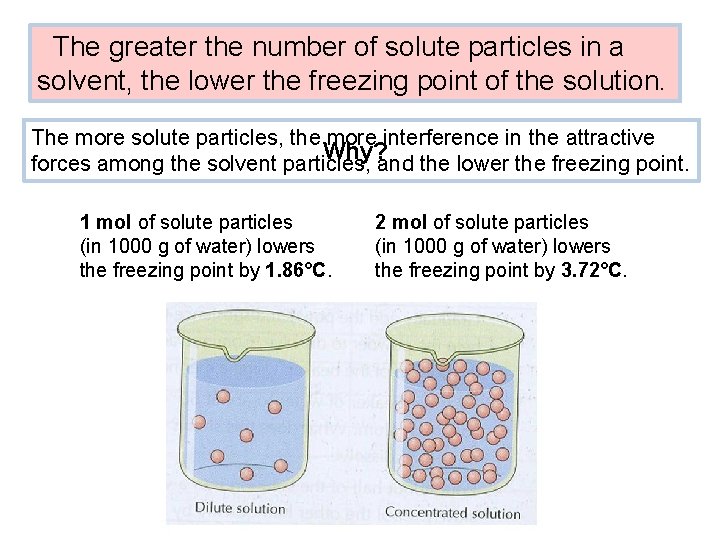
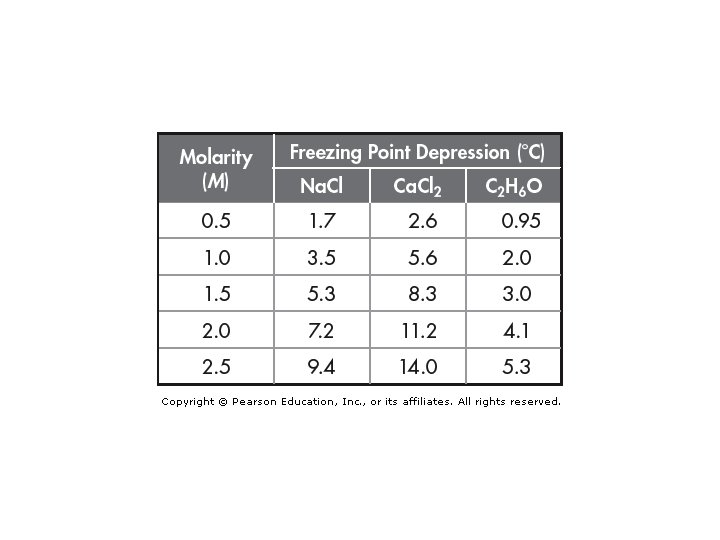
The greater the number of solute particles in a solvent, the lower the freezing point of the solution. The more solute particles, the more interference in the attractive Why? forces among the solvent particles, and the lower the freezing point. 1 mol of solute particles (in 1000 g of water) lowers the freezing point by 1. 86°C. 2 mol of solute particles (in 1000 g of water) lowers the freezing point by 3. 72°C.


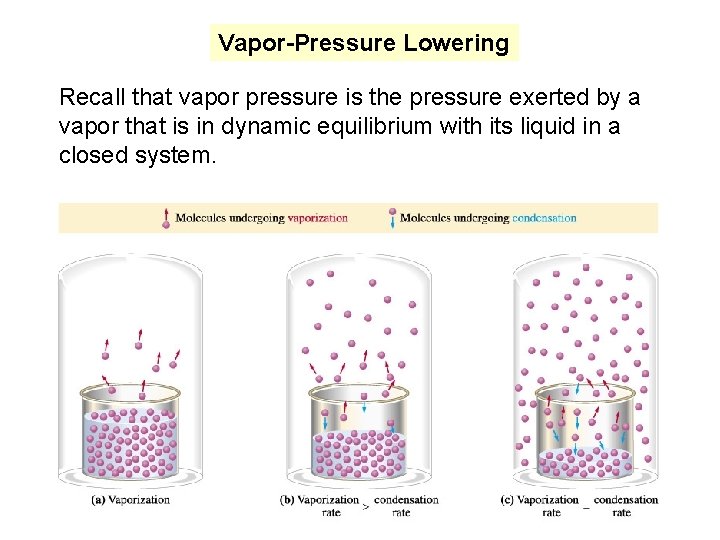
Vapor-Pressure Lowering Recall that vapor pressure is the pressure exerted by a vapor that is in dynamic equilibrium with its liquid in a closed system.

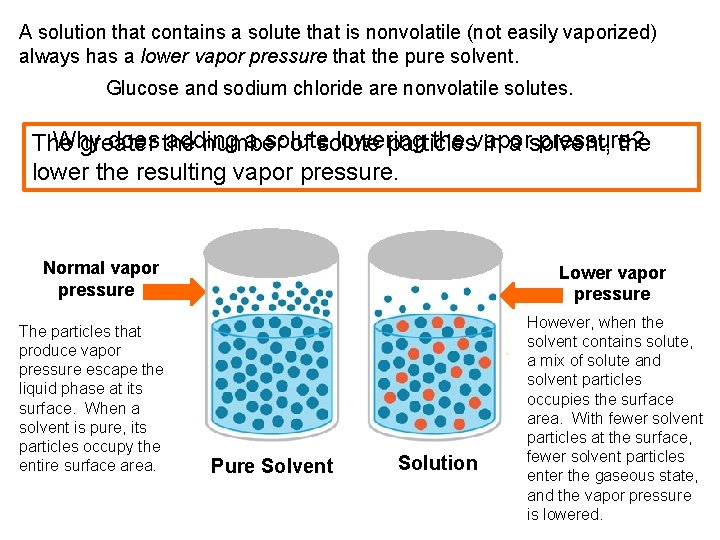
A solution that contains a solute that is nonvolatile (not easily vaporized) always has a lower vapor pressure that the pure solvent. Glucose and sodium chloride are nonvolatile solutes. Why does the adding a solute lowering the vapor pressure? The greater number of solute particles in a solvent, the lower the resulting vapor pressure. Normal vapor pressure The particles that produce vapor pressure escape the liquid phase at its surface. When a solvent is pure, its particles occupy the entire surface area. Lower vapor pressure Pure Solvent Solution However, when the solvent contains solute, a mix of solute and solvent particles occupies the surface area. With fewer solvent particles at the surface, fewer solvent particles enter the gaseous state, and the vapor pressure is lowered.

In an aqueous solution of glucose, a portion of the surface area is occupied by nonvolatile glucose molecules rather than by volatile water molecules. As a result, fewer water molecules can enter the vapor phase per unit time, even though the surface water molecules have the same kinetic energy distribution as they would in pure water. At the same time, the rate at which water molecules in the vapor phase collide with the surface and reenter the solution is unaffected. The net effect is to shift the dynamic equilibrium between water in the vapor and the liquid phases, decreasing the vapor pressure of the solution compared with the vapor pressure of the pure solvent. SKIP

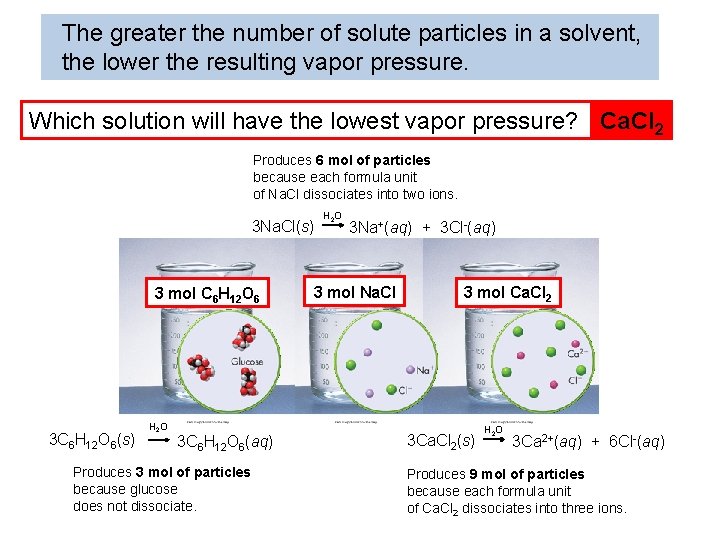
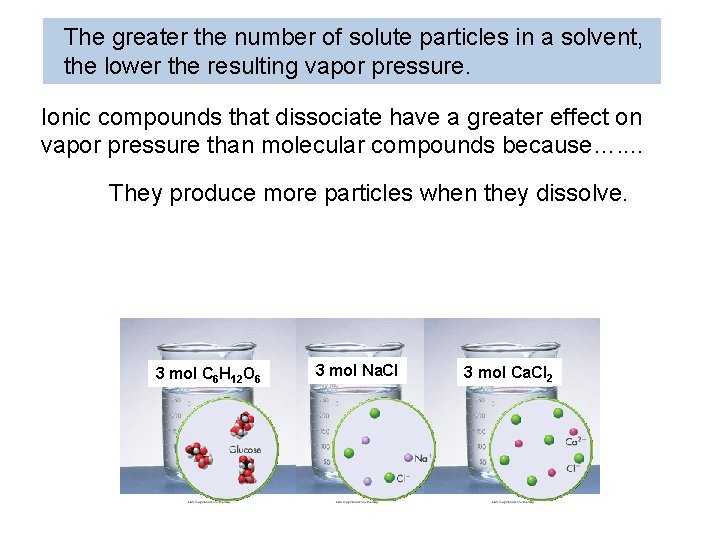
The greater the number of solute particles in a solvent, the lower the resulting vapor pressure. Which solution will have the lowest vapor pressure? Ca. Cl 2 Produces 6 mol of particles because each formula unit of Na. Cl dissociates into two ions. 3 Na. Cl(s) 3 mol C 6 H 12 O 6 3 C 6 H 12 O 6(s) H 2 O 3 C 6 H 12 O 6(aq) Produces 3 mol of particles because glucose does not dissociate. H 2 O 3 Na+(aq) + 3 Cl-(aq) 3 mol Na. Cl 3 mol Ca. Cl 2 3 Ca. Cl 2(s) H 2 O 3 Ca 2+(aq) + 6 Cl-(aq) Produces 9 mol of particles because each formula unit of Ca. Cl 2 dissociates into three ions.

The greater the number of solute particles in a solvent, the lower the resulting vapor pressure. Ionic compounds that dissociate have a greater effect on vapor pressure than molecular compounds because…. . They produce more particles when they dissolve. 3 mol C 6 H 12 O 6 3 mol Na. Cl 3 mol Ca. Cl 2


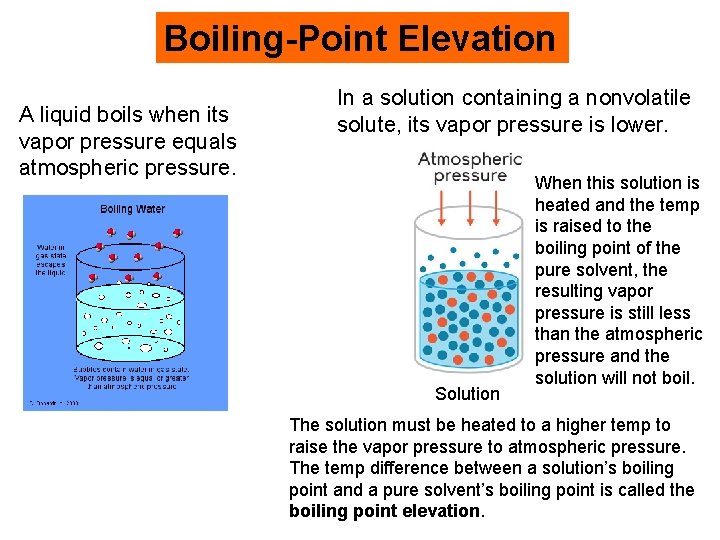
Boiling-Point Elevation A liquid boils when its vapor pressure equals atmospheric pressure. In a solution containing a nonvolatile solute, its vapor pressure is lower. Solution When this solution is heated and the temp is raised to the boiling point of the pure solvent, the resulting vapor pressure is still less than the atmospheric pressure and the solution will not boil. The solution must be heated to a higher temp to raise the vapor pressure to atmospheric pressure. The temp difference between a solution’s boiling point and a pure solvent’s boiling point is called the boiling point elevation.

The fluid circulating through a car’s cooling system is a solution of water and ethylene glycol, or antifreeze. Ethylene glycol raising the boiling point of the solution to above the boiling point of water. This helps protect the car from overheating in the summer.

https: //www. youtube. com/watch? v=F 8 dyc-t. MGbw https: //www. youtube. com/watch? v=5 m 8 qv. QHdxu. A&ebc=ANy. Px. Kok. Pzj ev 67 l. Aswqbi. Vuf. JOb. Ej. J 5 aevl. TTw. Tsuydwzptfpv. I 20 L 81 mf. A 5 NQlr. S 5 Gl. Pj ja 4 r. UEfhf 8 vy 1 QR 1 f. OB 183 Qra. ZA https: //www. youtube. com/watch? v=Jkh. WV 2 ua. Ha. A

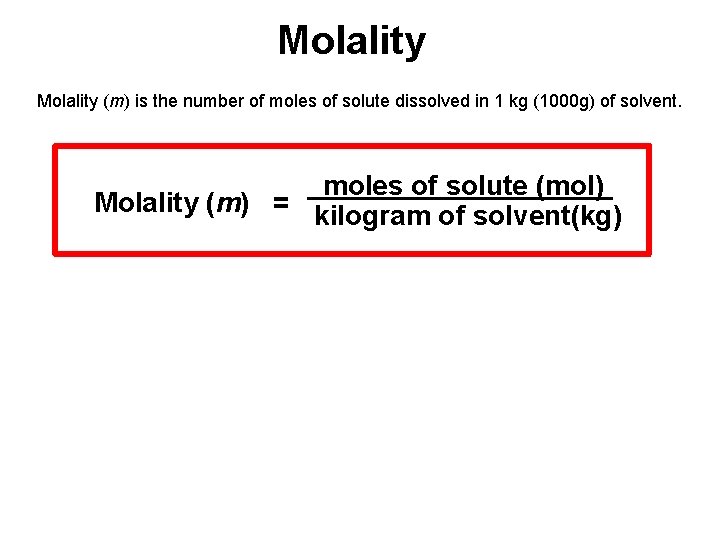
Molality (m) is the number of moles of solute dissolved in 1 kg (1000 g) of solvent. moles of solute (mol) Molality (m) = kilogram of solvent(kg)

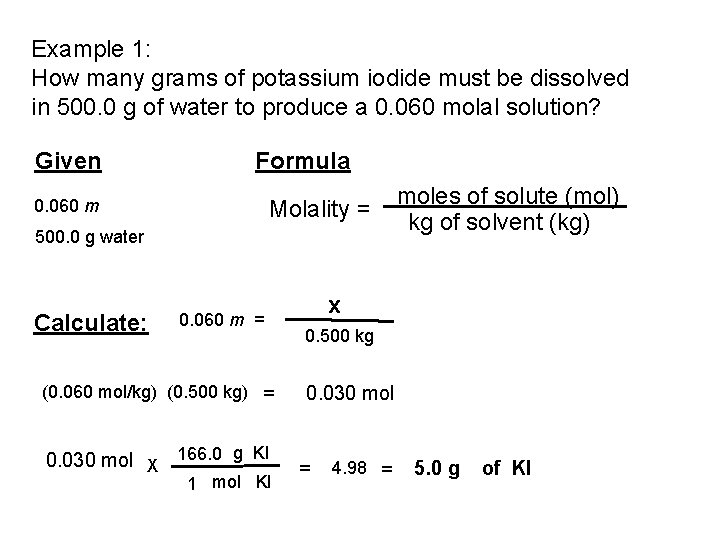
Example 1: How many grams of potassium iodide must be dissolved in 500. 0 g of water to produce a 0. 060 molal solution? Given Formula 0. 060 m Molality = 500. 0 g water Calculate: 0. 060 m = (0. 060 mol/kg) (0. 500 kg) = 0. 030 mol X 166. 0 g KI 1 mol KI moles of solute (mol) kg of solvent (kg) x 0. 500 kg 0. 030 mol = 4. 98 = 5. 0 g of KI

The magnitudes of the freezing-point depression (∆Tf) and the boiling-point elevation (∆Tb) of a solution are directly proportional to the molal conc (m), assuming the solute is molecular, not ionic. ∆Tf = Kf X m ∆Tb = Kb X m

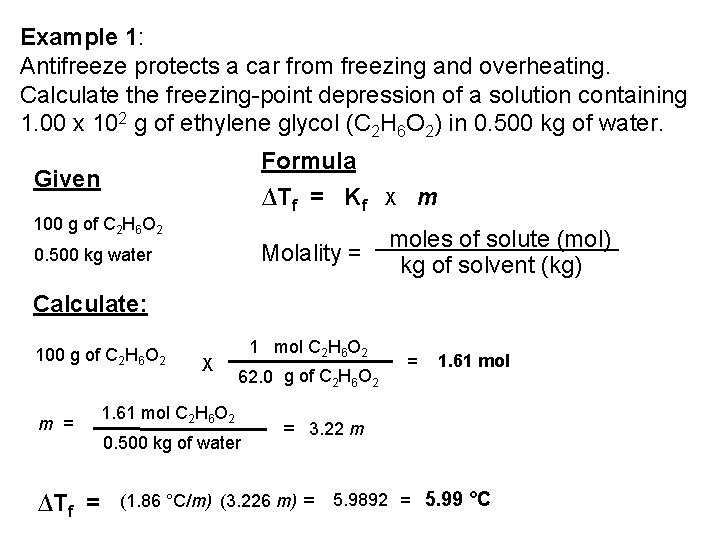
Example 1: Antifreeze protects a car from freezing and overheating. Calculate the freezing-point depression of a solution containing 1. 00 x 102 g of ethylene glycol (C 2 H 6 O 2) in 0. 500 kg of water. Formula Given 100 g of C 2 H 6 O 2 0. 500 kg water ∆Tf = Kf X m Molality = moles of solute (mol) kg of solvent (kg) Calculate: 100 g of C 2 H 6 O 2 m = ∆Tf = X 1 mol C 2 H 6 O 2 62. 0 g of C 2 H 6 O 2 1. 61 mol C 2 H 6 O 2 0. 500 kg of water = 1. 61 mol = 3. 22 m (1. 86 °C/m) (3. 226 m) = 5. 9892 = 5. 99 °C