7 6 NOTES Metal Reactivity Trends Metal Reactivity














- Slides: 17

7. 6 – NOTES Metal Reactivity Trends

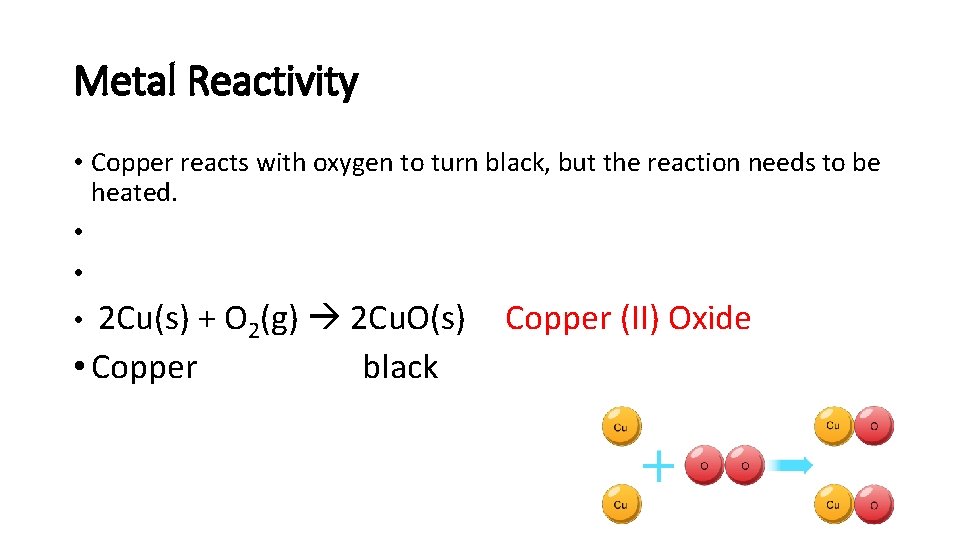
Metal Reactivity • Copper reacts with oxygen to turn black, but the reaction needs to be heated. • • 2 Cu(s) + O 2(g) 2 Cu. O(s) • Copper black • Copper (II) Oxide

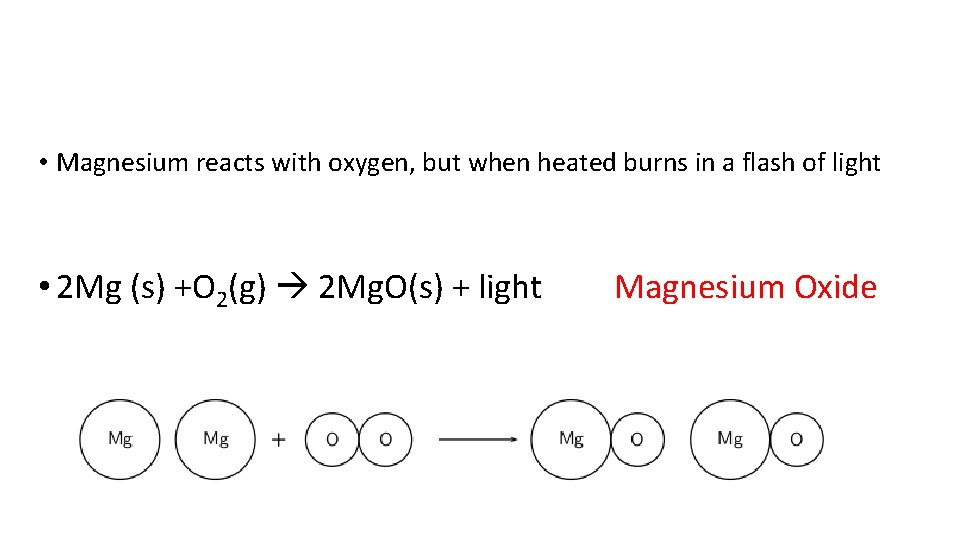
• Magnesium reacts with oxygen, but when heated burns in a flash of light • 2 Mg (s) +O 2(g) 2 Mg. O(s) + light Magnesium Oxide

• Gold will not react with oxygen, even when heated • Different metals have different levels of reactivity, just as you saw in the Relative Reactivities of Metals Lab • How would you rank the reactivities of Cu, Mg and Au? • Most Mg, Cu, Au least

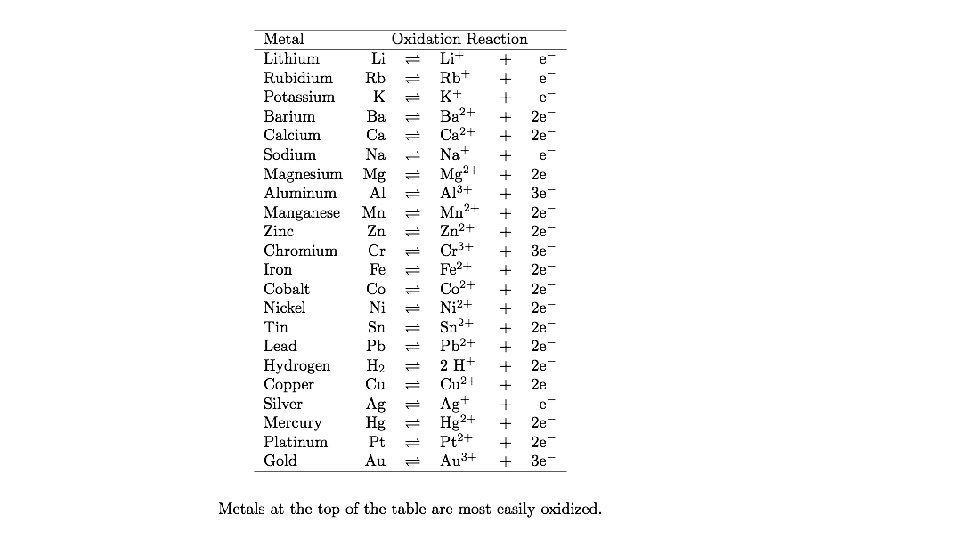
• Activity series • Ranking the elements according to their chemical reactivities • See handout • The activity series predicts if chemical reactions will occur • Most reactive at top • Least reactive at bottom


How do we know if a reaction will occur? • By looking at relative reactivities on the Metal Activity Series. • 1. Identify if the reaction will occur. 2. Predict the Products. • 1. K + Ag 2 S • • • 2. Br 2 + Na. Cl • • • 3. Pb (IV) + Ni. Cl 2

• Use your activity series and periodic table to answer the following questions 1. Will Pb metal replace Ag+ ions? 1. Yes, Pb is above Ag, it is more reactive

• 2. What trend in metallic reactivity is found as you move from left to right across a horizontal row (period) of the periodic table? (Hint: compare the reactivity of sodium with magnesium and aluminum. ) • Metal reactivity goes down as you move L to R

• 3. Where are the most reactive metals on the periodic table found? • Top left corner alkali and alkaline earth metals

• 4. Where are the least reactive metals located? • Transition Metals

• 5. Will iron (Fe) react with a solution of lead (II) nitrate (Pb(NO 3)2)? • Fe + PB(NO 3)2 yes, reactions happens, Fe is above Pb

• 6. Will platinum (Pt) metal react with a lead (II) nitrate solution? • Pt + Pb(NO 3)2 no, Pt is below Pb

• 7. Explain your answers to questions 5 and 6. • Element is above ion, reaction will happen • Element is below ion, reaction won’t happen

• Use specific examples from the activity series in your answers to these two questions: • Are the least reactive metals also the least expensive metals? • No, Au, Pt, Ag are least reactive but expensive.

• If not, what other factor(s) might influence the market value of a metal? • Abundance, fashion, preference, strength, how easily is it mined.

Examples from activity.