Section 5 3 Periodicity Trends PERIODICITY trend in




- Slides: 40

Section 5. 3 Periodicity Trends

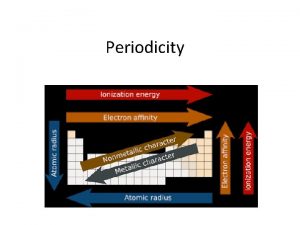
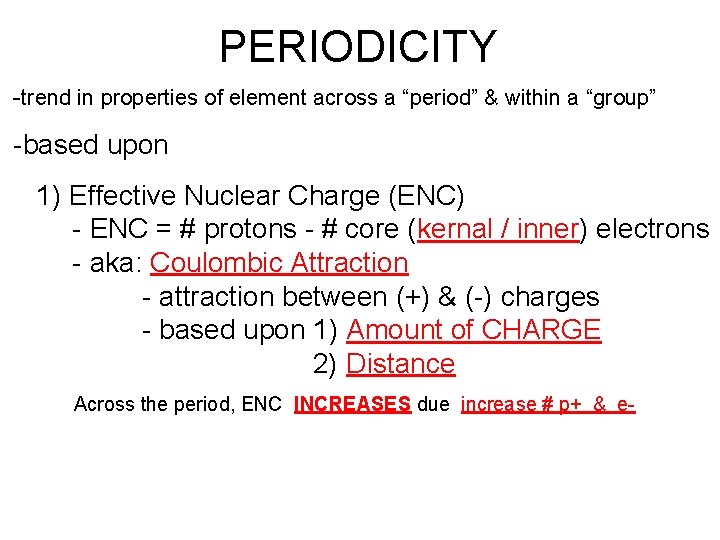
PERIODICITY -trend in properties of element across a “period” & within a “group” -based upon 1) Effective Nuclear Charge (ENC) - ENC = # protons - # core (kernal / inner) electrons - aka: Coulombic Attraction - attraction between (+) & (-) charges - based upon 1) Amount of CHARGE 2) Distance Across the period, ENC INCREASES due increase # p+ & e-

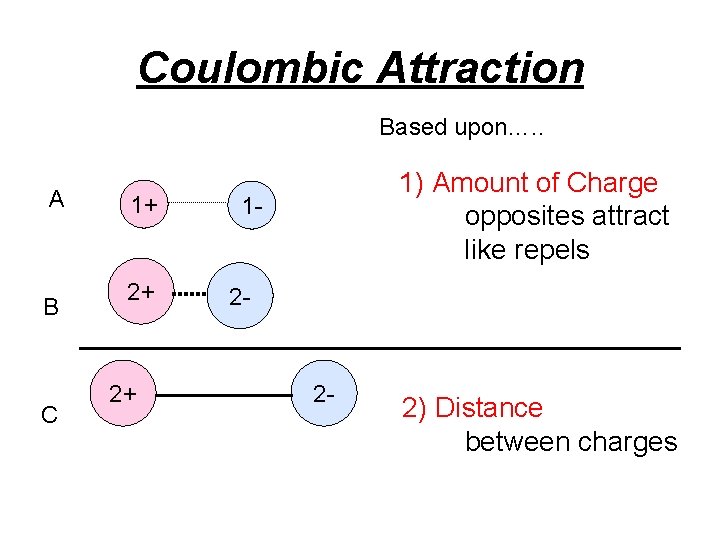
Coulombic Attraction Based upon…. . A B C 1+ 2+ 2+ 1) Amount of Charge opposites attract like repels 1 - 2 - 2 - 2) Distance between charges

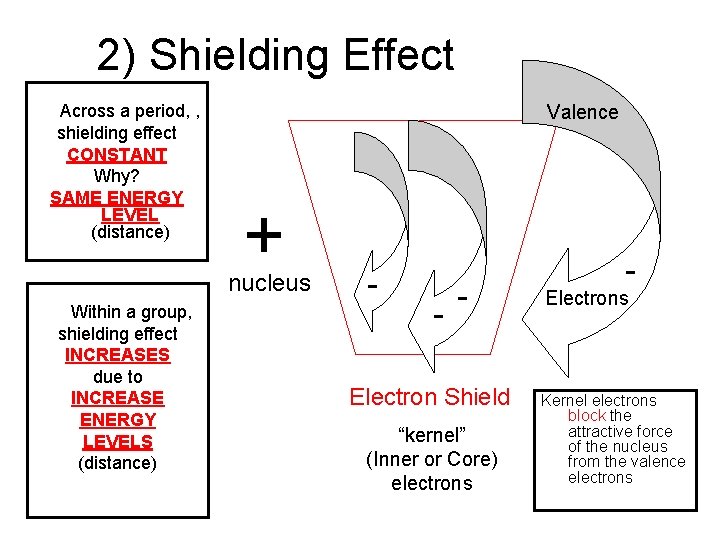
2) Shielding Effect Across a period, , shielding effect CONSTANT Why? SAME ENERGY LEVEL (distance) Valence + nucleus Within a group, shielding effect INCREASES due to INCREASE ENERGY LEVELS (distance) - - - Electron Shield “kernel” (Inner or Core) electrons - Electrons Kernel electrons block the attractive force of the nucleus from the valence electrons

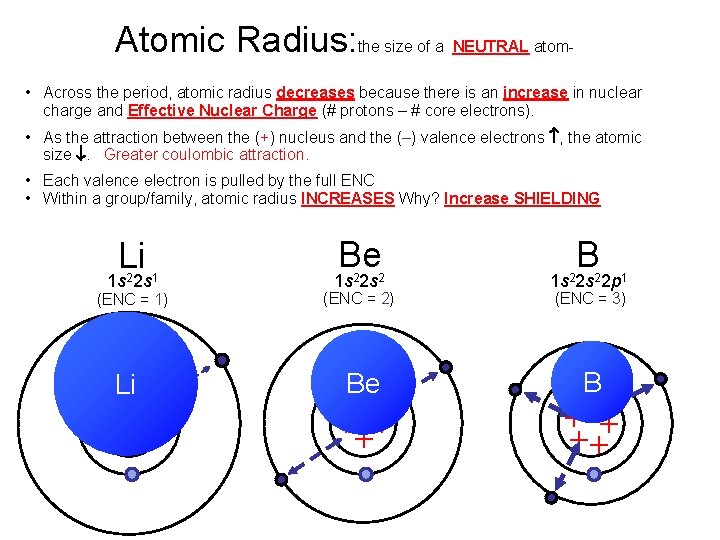
Atomic Radius: the size of a NEUTRAL atom • Across the period, atomic radius decreases because there is an increase in nuclear charge and Effective Nuclear Charge (# protons – # core electrons). • As the attraction between the (+) nucleus and the (–) valence electrons , the atomic size . Greater coulombic attraction. • Each valence electron is pulled by the full ENC • Within a group/family, atomic radius INCREASES Why? Increase SHIELDING Li 1 s 22 s 1 (ENC = 1) Li ++ + Be B 1 s 22 s 22 p 1 Be B (ENC = 2) ++ + + (ENC = 3) +++ ++

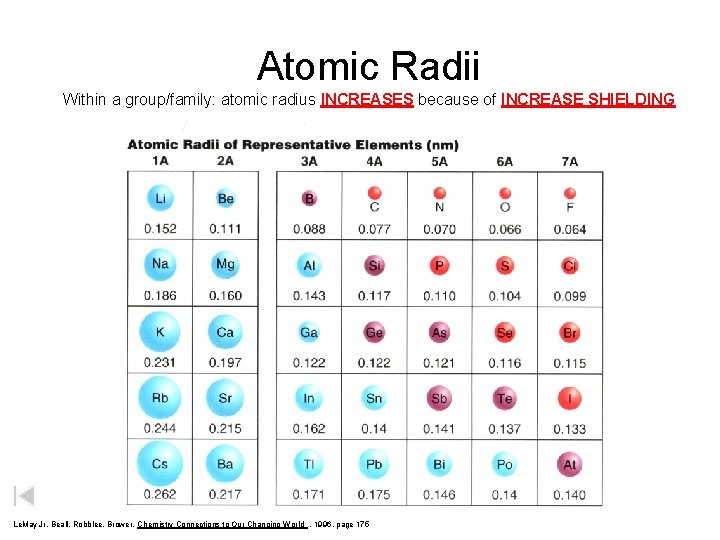
Atomic Radii Within a group/family: atomic radius INCREASES because of INCREASE SHIELDING Le. May Jr, Beall, Robblee, Brower, Chemistry Connections to Our Changing World , 1996, page 175

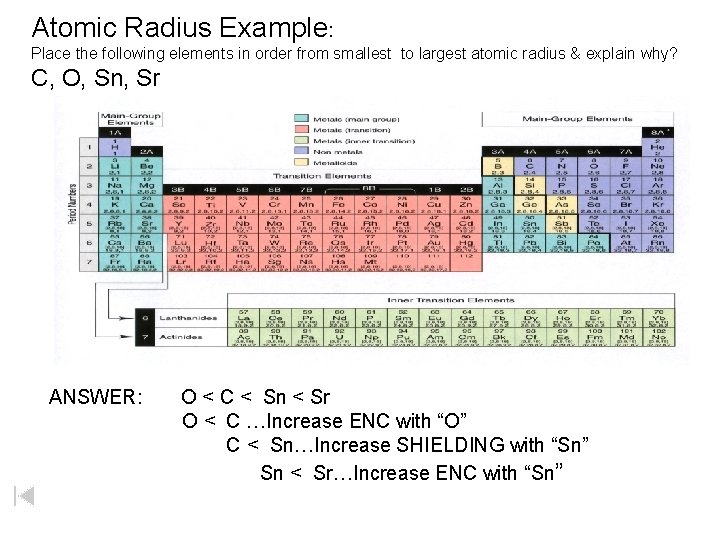
Atomic Radius Example: Place the following elements in order from smallest to largest atomic radius & explain why? C, O, Sn, Sr ANSWER: O < C < Sn < Sr O < C …Increase ENC with “O” C < Sn…Increase SHIELDING with “Sn” Sn < Sr…Increase ENC with “Sn”

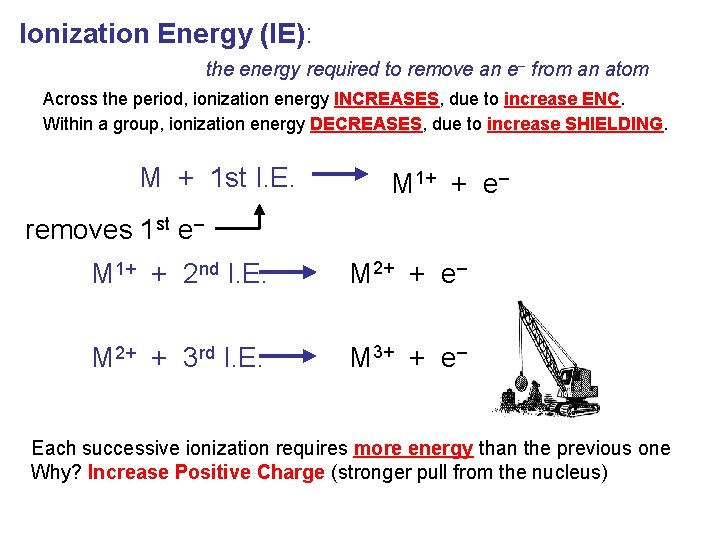
Ionization Energy (IE): the energy required to remove an e– from an atom Across the period, ionization energy INCREASES, due to increase ENC. Within a group, ionization energy DECREASES, due to increase SHIELDING. M + 1 st I. E. M 1+ + e– removes 1 st e– M 1+ + 2 nd I. E. M 2+ + e– M 2+ + 3 rd I. E. M 3+ + e– Each successive ionization requires more energy than the previous one Why? Increase Positive Charge (stronger pull from the nucleus)

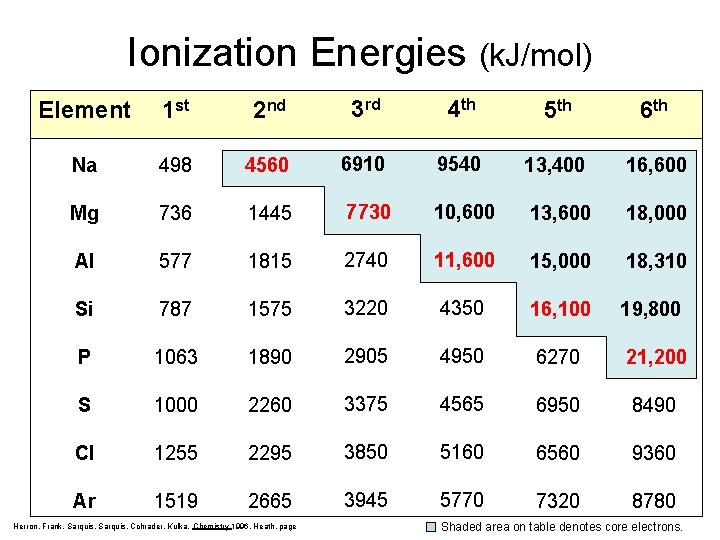
Ionization Energies (k. J/mol) Element 1 st 2 nd 3 rd 4 th 5 th 6 th Na 498 4560 6910 9540 13, 400 16, 600 Mg 736 1445 7730 10, 600 13, 600 18, 000 Al 577 1815 2740 11, 600 15, 000 18, 310 Si 787 1575 3220 4350 16, 100 19, 800 P 1063 1890 2905 4950 6270 21, 200 S 1000 2260 3375 4565 6950 8490 Cl 1255 2295 3850 5160 6560 9360 Ar 1519 2665 3945 5770 7320 8780 Herron, Frank, Sarquis, Cchrader, Kulka, Chemistry 1996, Heath, page Shaded area on table denotes core electrons.

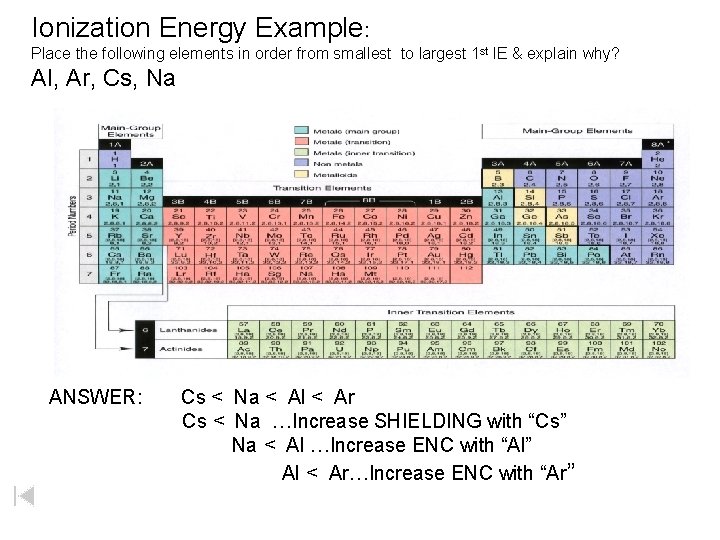
Ionization Energy Example: Place the following elements in order from smallest to largest 1 st IE & explain why? Al, Ar, Cs, Na ANSWER: Cs < Na < Al < Ar Cs < Na …Increase SHIELDING with “Cs” Na < Al …Increase ENC with “Al” Al < Ar…Increase ENC with “Ar”

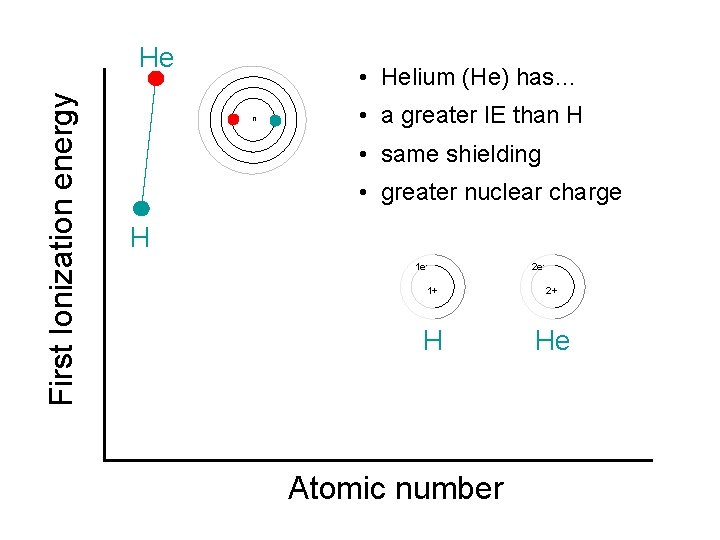
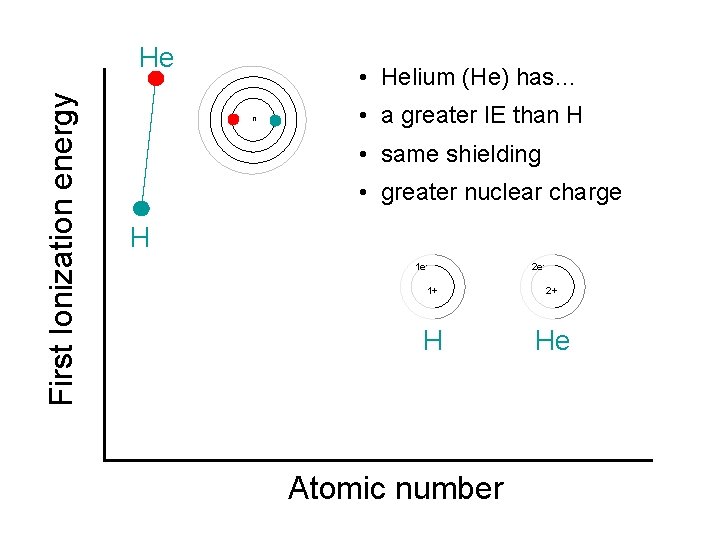
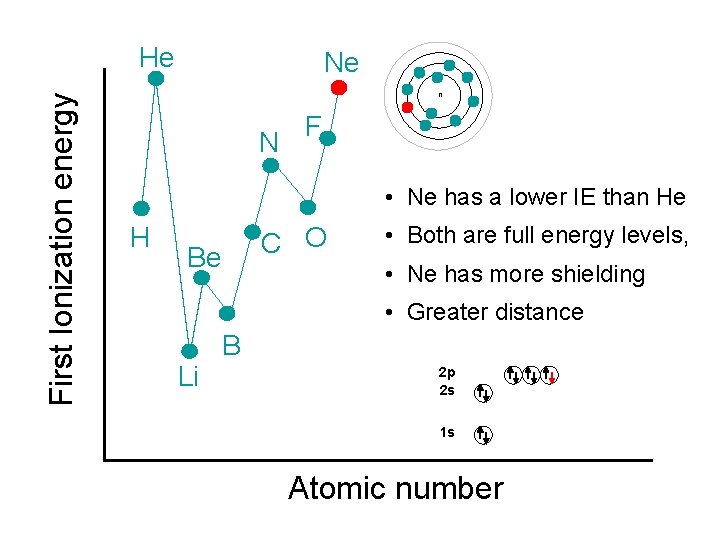
First Ionization energy He • Helium (He) has… n • a greater IE than H • same shielding • greater nuclear charge H 1 e- 2 e- 1+ 2+ H He Atomic number

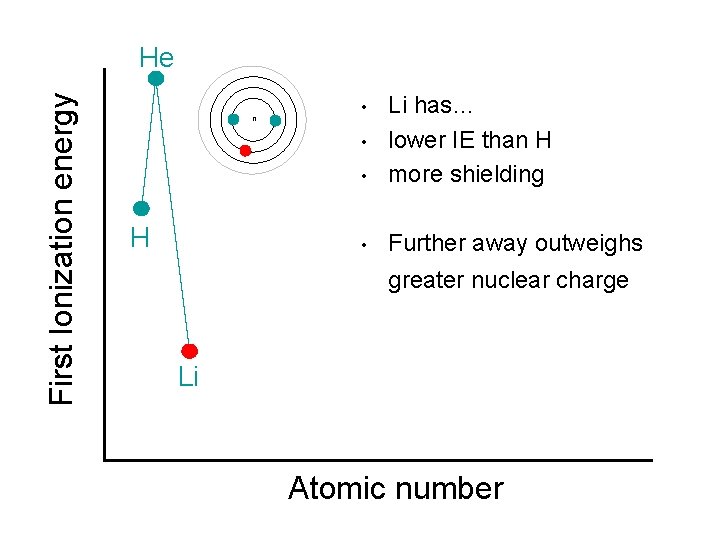
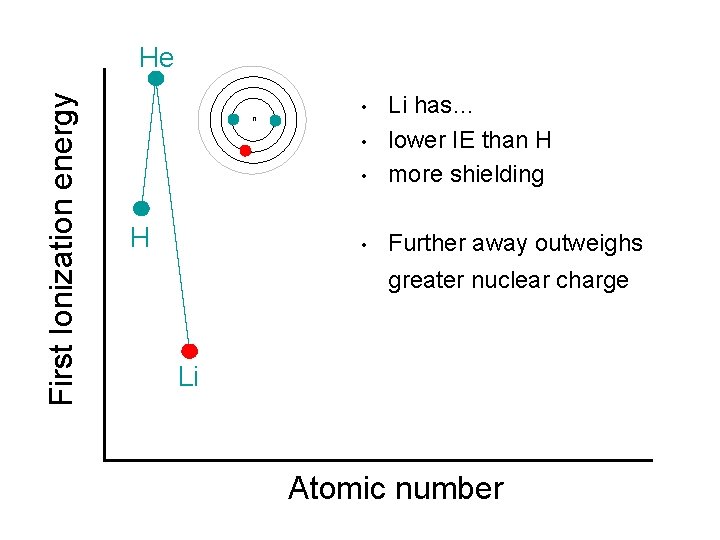
First Ionization energy He n • Li has… lower IE than H more shielding • Further away outweighs • • H greater nuclear charge Li Atomic number

First Ionization energy He • Helium (He) has… n • a greater IE than H • same shielding • greater nuclear charge H 1 e- 2 e- 1+ 2+ H He Atomic number

First Ionization energy He n • Li has… lower IE than H more shielding • Further away outweighs • • H greater nuclear charge Li Atomic number

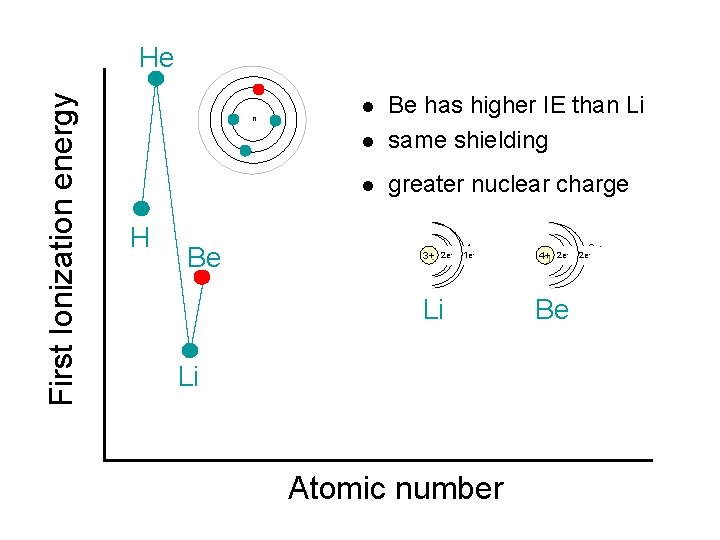
First Ionization energy He n H l Be has higher IE than Li same shielding l greater nuclear charge l 2 e- Be 2 e 1 e- - 3+ 3+ 2 e 1 e Li Li Atomic number 2 e 4+4+2 e- Be

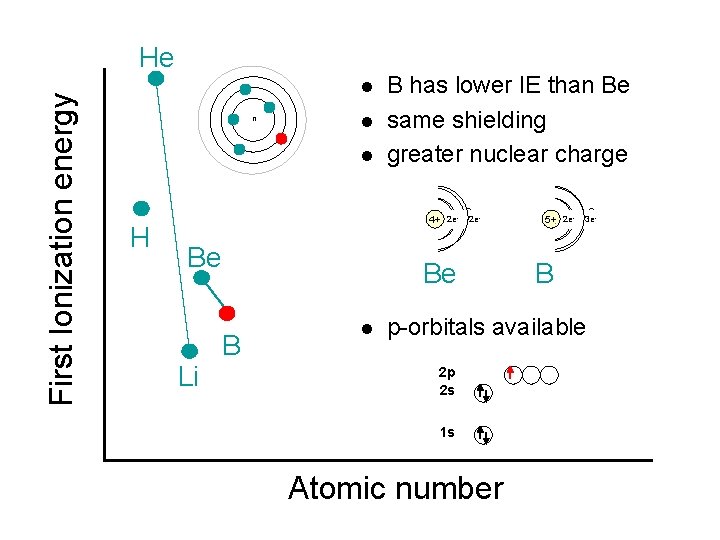
First Ionization energy He l n l l B has lower IE than Be same shielding greater nuclear charge 2 e- 2 e 2 e-- H 4+ 2 e- 2 e 4+ Be Li Be B l 3 e- 5+ 5+ 2 e 3 e B p-orbitals available 2 p 2 s 1 s Atomic number

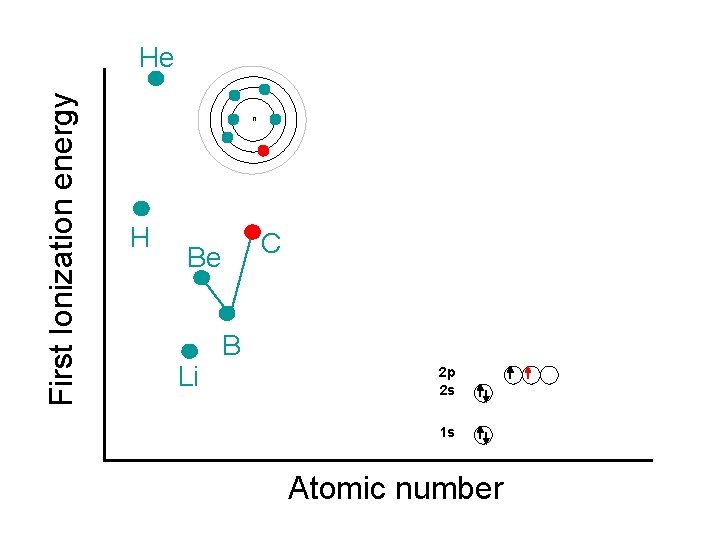
First Ionization energy He n H C Be Li B 2 p 2 s 1 s Atomic number

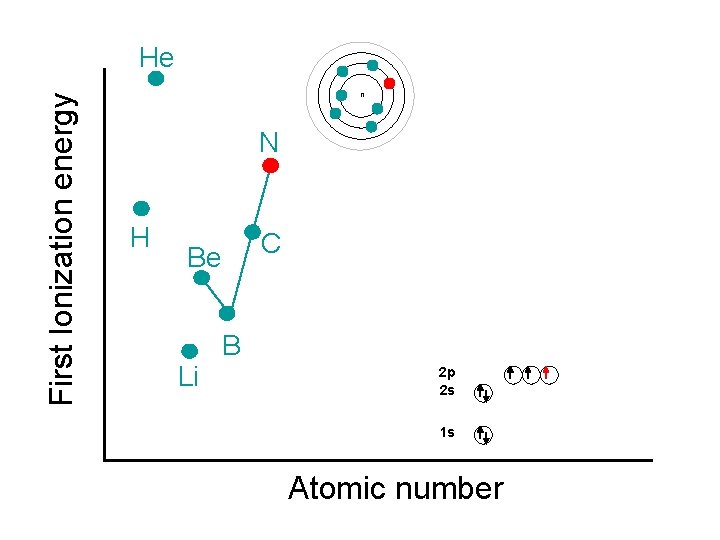
First Ionization energy He n N H C Be Li B 2 p 2 s 1 s Atomic number

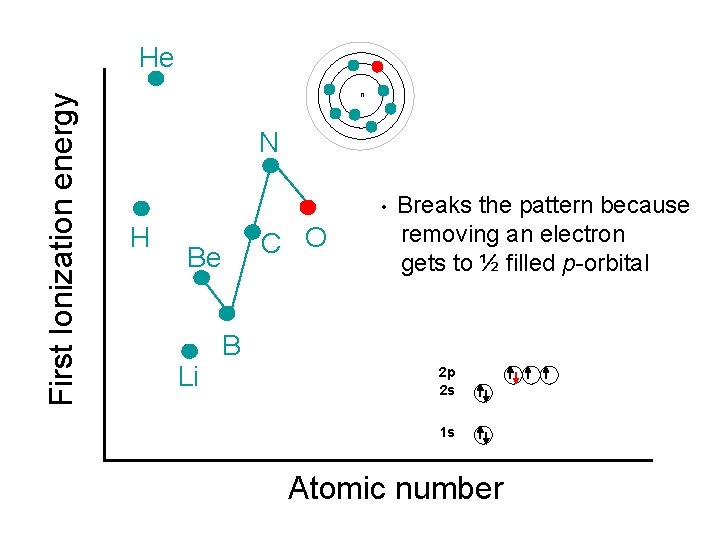
First Ionization energy He n N • H C O Be Li Breaks the pattern because removing an electron gets to ½ filled p-orbital B 2 p 2 s 1 s Atomic number

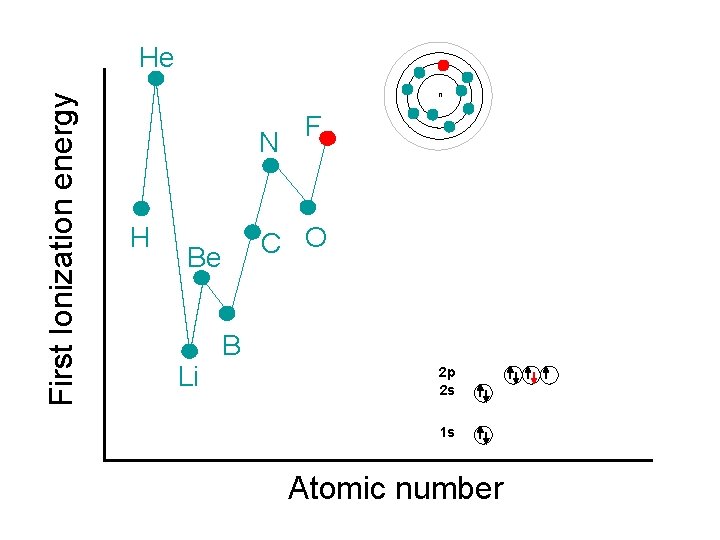
First Ionization energy He n N H C O Be Li F B 2 p 2 s 1 s Atomic number

First Ionization energy He Ne n N F • Ne has a lower IE than He H C O Be • Both are full energy levels, • Ne has more shielding • Greater distance Li B 2 p 2 s 1 s Atomic number

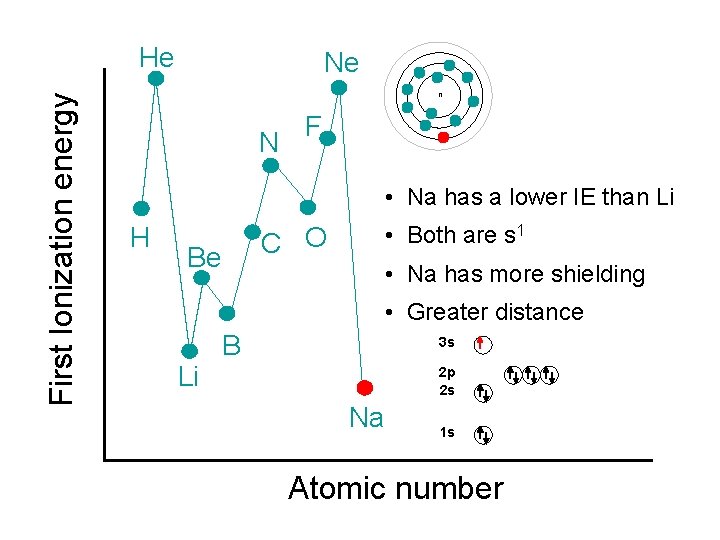
First Ionization energy He Ne n N F • Na has a lower IE than Li H C O Be • Both are s 1 • Na has more shielding • Greater distance Li B 3 s 2 p 2 s Na 1 s Atomic number

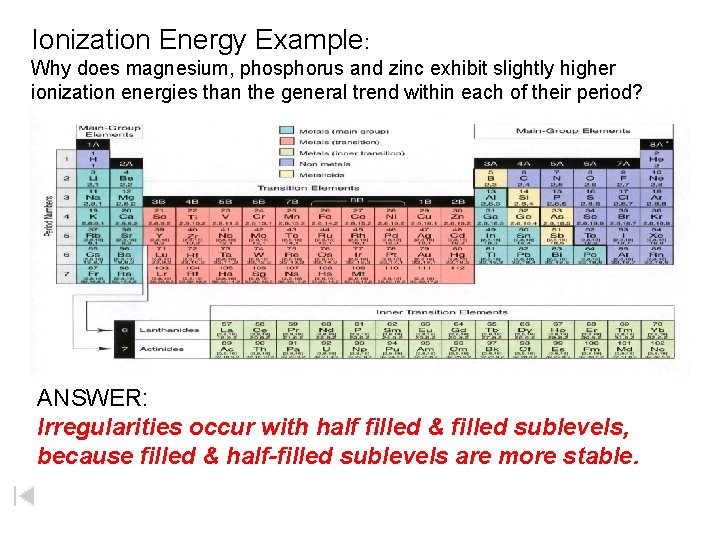
Ionization Energy Example: Why does magnesium, phosphorus and zinc exhibit slightly higher ionization energies than the general trend within each of their period? ANSWER: Irregularities occur with half filled & filled sublevels, because filled & half-filled sublevels are more stable.

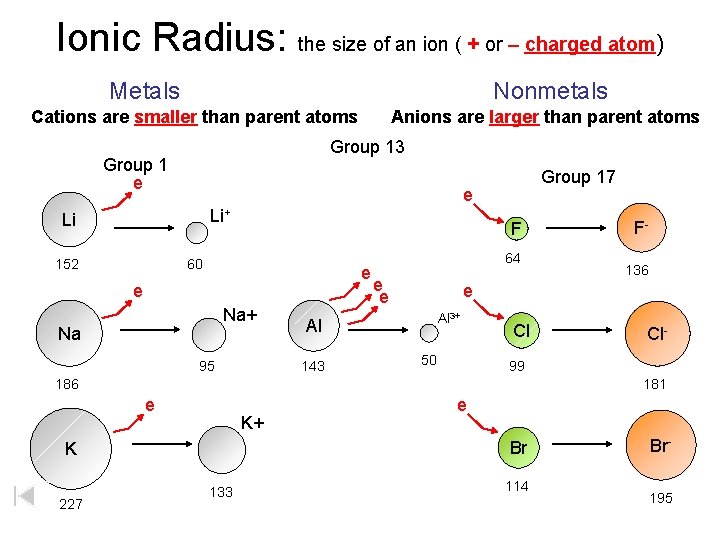
Ionic Radius: the size of an ion ( + or – charged atom) Metals Nonmetals Cations are smaller than parent atoms Anions are larger than parent atoms Group 13 Group 1 e e Li+ Li 152 Group 17 F 60 e e Na+ Na 95 64 e e Al 3+ 50 Cl Cl- 99 186 181 e K+ e Br K 227 136 e Al 143 F- 133 114 Br 195

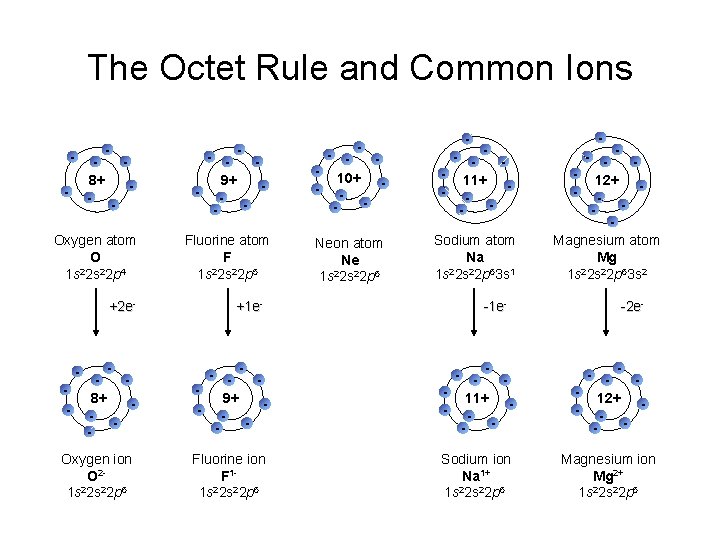
The Octet Rule and Common Ions - - 8+ - - Oxygen atom O 2 1 s 2 s 22 p 4 - - 9+ - - 10+ - - 11+ - - Fluorine atom F 2 1 s 2 s 22 p 5 Neon atom Ne 2 1 s 2 s 22 p 6 Sodium atom Na 2 1 s 2 s 22 p 63 s 1 - - 12+ - Magnesium atom Mg 2 1 s 2 s 22 p 63 s 2 +2 e- +1 e- 8+ - - 9+ - - 11+ - - 12+ - - Oxygen ion O 21 s 22 p 6 Fluorine ion F 11 s 22 p 6 Sodium ion Na 1+ 1 s 22 p 6 Magnesium ion Mg 2+ 1 s 22 p 6 - -1 e- - -2 e-

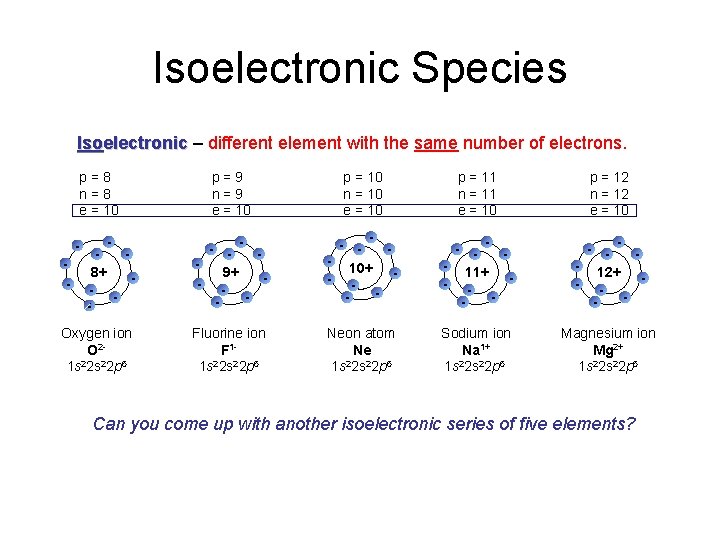
Isoelectronic Species Isoelectronic – different element with the same number of electrons. p=8 n=8 e = 10 p=9 n=9 e = 10 p = 10 n = 10 e = 10 p = 11 n = 11 e = 10 p = 12 n = 12 e = 10 8+ - - 9+ - - 10+ - - 11+ - - 12+ - - Oxygen ion O 21 s 22 p 6 Fluorine ion F 11 s 22 p 6 Neon atom Ne 2 1 s 2 s 22 p 6 Sodium ion Na 1+ 1 s 22 p 6 Magnesium ion Mg 2+ 1 s 22 p 6 - - Can you come up with another isoelectronic series of five elements?

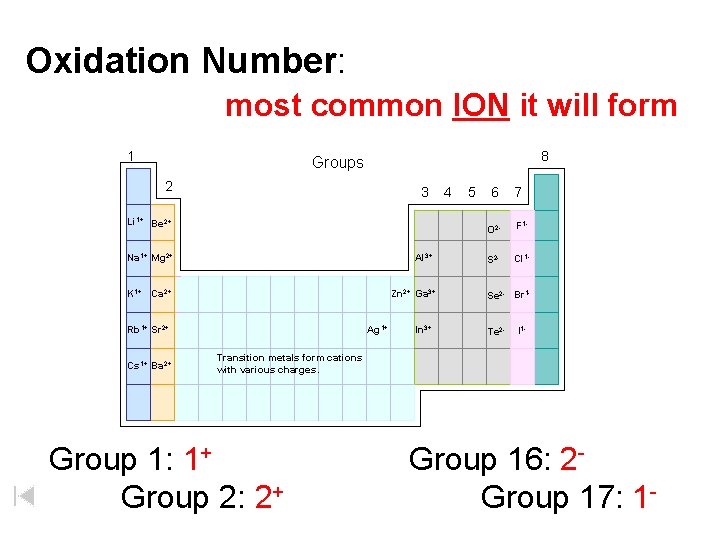
Oxidation Number: most common ION it will form 1 8 Groups 2 3 Li 1+ Be 2+ Na 1+ Mg 2+ Al 3+ K 1+ Ca 2+ Zn 2+ Ga 3+ Rb 1+ Sr 2+ Cs 1+ Ba 2+ Ag 1+ In 3+ 4 5 6 7 O 2 - F 1 - S 2 - Cl 1 - Se 2 - Br 1 Te 2 - I 1 - Transition metals form cations with various charges. Group 1: 1+ Group 2: 2+ Group 16: 2 Group 17: 1 -

Stop

What is “PERIODICITY”? Periodicity is predictable repeating patterns/trends on the periodic table.

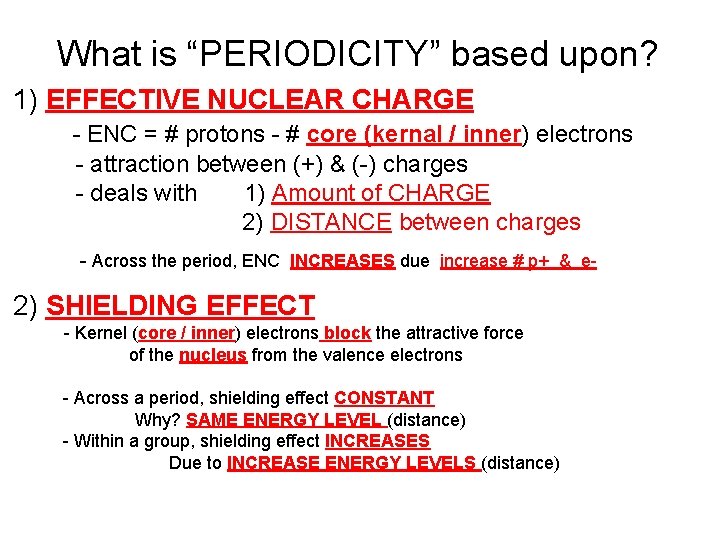
What is “PERIODICITY” based upon? 1) EFFECTIVE NUCLEAR CHARGE - ENC = # protons - # core (kernal / inner) electrons - attraction between (+) & (-) charges - deals with 1) Amount of CHARGE 2) DISTANCE between charges - Across the period, ENC INCREASES due increase # p+ & e- 2) SHIELDING EFFECT - Kernel (core / inner) electrons block the attractive force of the nucleus from the valence electrons - Across a period, shielding effect CONSTANT Why? SAME ENERGY LEVEL (distance) - Within a group, shielding effect INCREASES Due to INCREASE ENERGY LEVELS (distance)

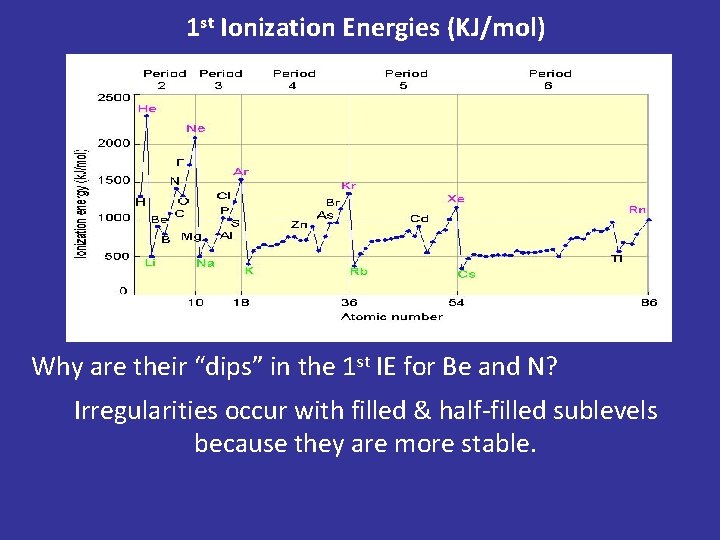
1 st Ionization Energies (KJ/mol) Why are their “dips” in the 1 st IE for Be and N? Irregularities occur with filled & half-filled sublevels because they are more stable.

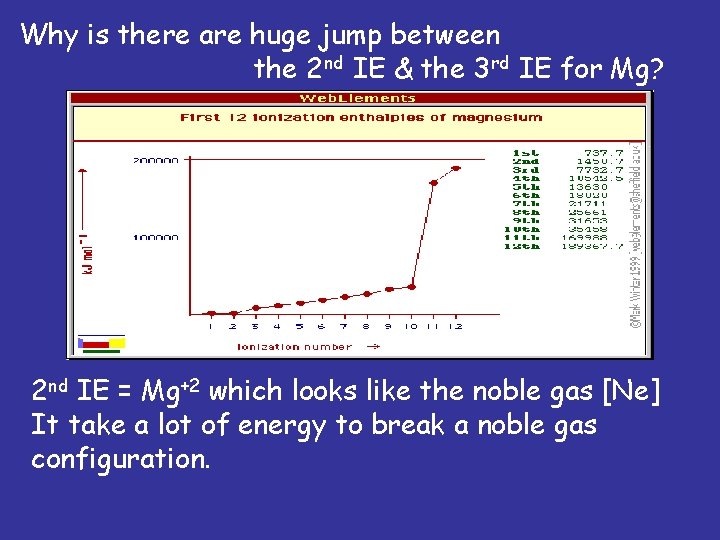
Why is there are huge jump between the 2 nd IE & the 3 rd IE for Mg? 2 nd IE = Mg+2 which looks like the noble gas [Ne] It take a lot of energy to break a noble gas configuration.

Electronegativity A measure ability of an atom in a chemical compound to attract electrons Across a period, EN’s INCREASE WHY? Increase Nuclear Charge Within a group/family, EN’s DECREASE or remain the same WHY? Increase Shielding

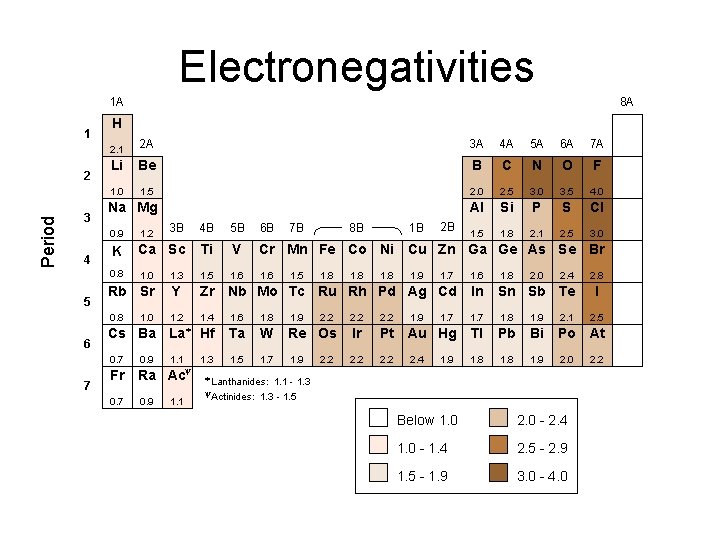
Electronegativities 1 A 1 Period 2 3 4 5 6 7 8 A H 2. 1 2 A 3 A 4 A 5 A 6 A 7 A Li Be B C N O F 1. 0 1. 5 2. 0 2. 5 3. 0 3. 5 4. 0 Al Si P S Cl 1. 5 1. 8 2. 1 2. 5 3. 0 Na Mg 1. 2 3 B 4 B 5 B 6 B K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 0. 8 1. 0 1. 3 1. 5 1. 6 1. 7 1. 6 1. 8 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te 0. 8 1. 2 1. 4 1. 6 1. 8 1. 9 2. 2 1. 7 1. 8 1. 9 2. 1 Cs Ba La* Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At 0. 7 1. 1 1. 3 1. 5 1. 7 1. 9 2. 2 1. 8 1. 9 2. 0 Fr 0. 7 1. 0 0. 9 y Ra Ac 0. 9 1. 1 8 B 7 B 1. 5 1. 8 2. 2 1. 8 1 B 2 B 0. 9 1. 8 1. 9 2. 4 1. 9 2. 0 2. 4 * Lanthanides: 1. 1 - 1. 3 - 1. 5 y. Actinides: Below 1. 0 2. 0 - 2. 4 1. 0 - 1. 4 2. 5 - 2. 9 1. 5 - 1. 9 3. 0 - 4. 0 2. 8 I 2. 5 2. 2

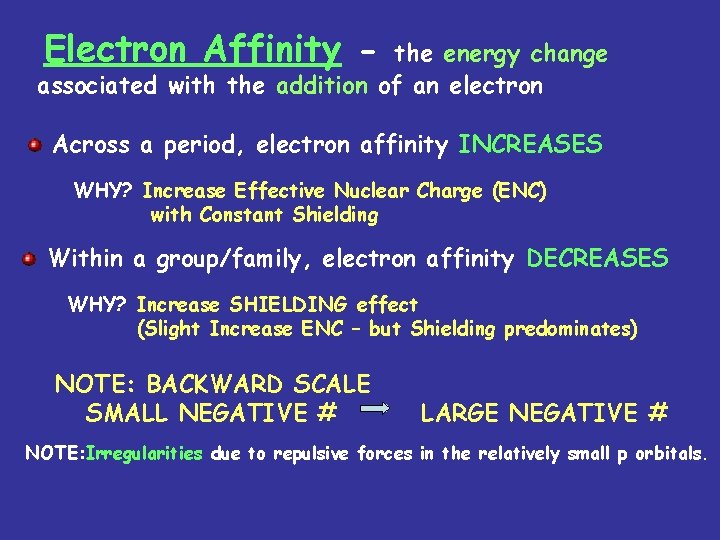
Electron Affinity - the energy change associated with the addition of an electron Across a period, electron affinity INCREASES WHY? Increase Effective Nuclear Charge (ENC) with Constant Shielding Within a group/family, electron affinity DECREASES WHY? Increase SHIELDING effect (Slight Increase ENC – but Shielding predominates) NOTE: BACKWARD SCALE SMALL NEGATIVE # LARGE NEGATIVE # NOTE: Irregularities due to repulsive forces in the relatively small p orbitals.

Table of Electron Affinities

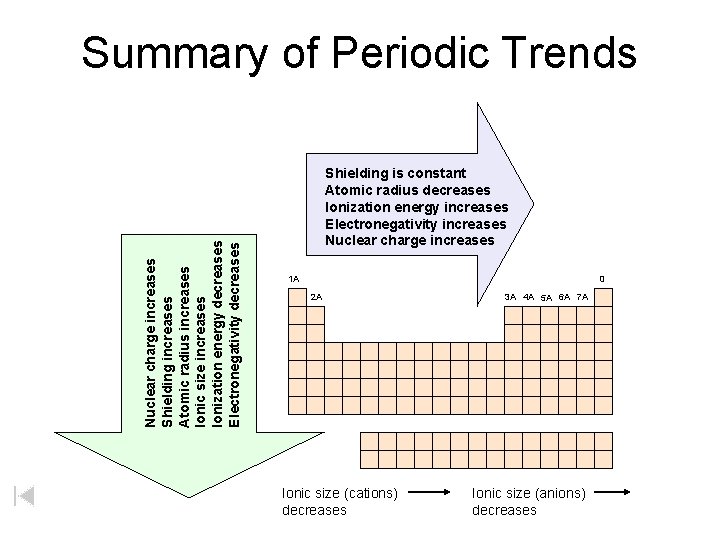
Nuclear charge increases Shielding increases Atomic radius increases Ionic size increases Ionization energy decreases Electronegativity decreases Summary of Periodic Trends Shielding is constant Atomic radius decreases Ionization energy increases Electronegativity increases Nuclear charge increases 1 A 0 2 A Ionic size (cations) decreases 3 A 4 A 5 A 6 A 7 A Ionic size (anions) decreases


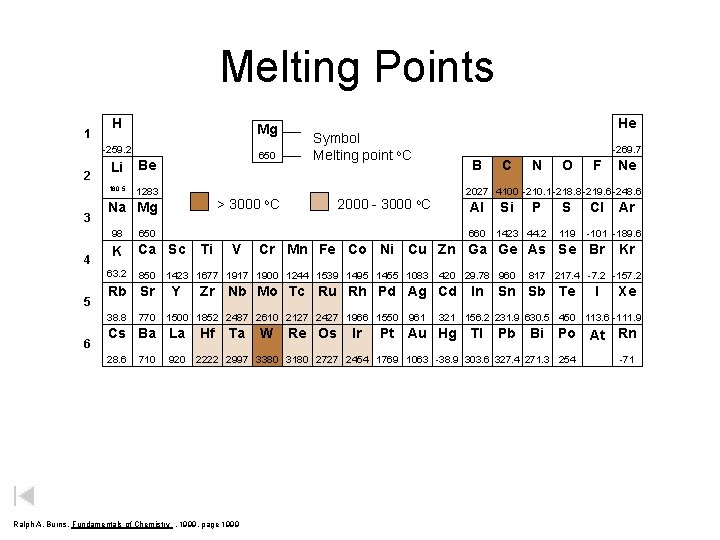
Melting Points 1 H Mg -259. 2 2 3 4 Li Be 180. 5 1283 98 650 K Ca Sc 850 Rb Sr 38. 8 6 > 3000 Na Mg 63. 2 5 650 770 710 o. C 2000 - 3000 -269. 7 B Ti V Al 1500 1852 2487 2610 2127 2427 1966 1550 920 Ta Si P 1423 44. 2 420 29. 78 960 Zr Nb Mo Tc Ru Rh Pd Ag Cd Hf N O S 119 W Re Os Ir 961 Ne Cl In Ar -101 -189. 6 Kr 817 217. 4 -7. 2 -157. 2 Sn Sb Te I Xe 321 156. 2 231. 9 630. 5 450 113. 6 -111. 9 Pt Au Hg Tl Pb Bi Po At Rn 2222 2997 3380 3180 2727 2454 1769 1063 -38. 9 303. 6 327. 4 271. 3 254 Ralph A. Burns, Fundamentals of Chemistry , 1999, page 1999 F Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br 1423 1677 1917 1900 1244 1539 1495 1455 1083 Y C 2027 4100 -210. 1 -218. 8 -219. 6 -248. 6 o. C 660 Cs Ba La 28. 6 He Symbol Melting point o. C -71

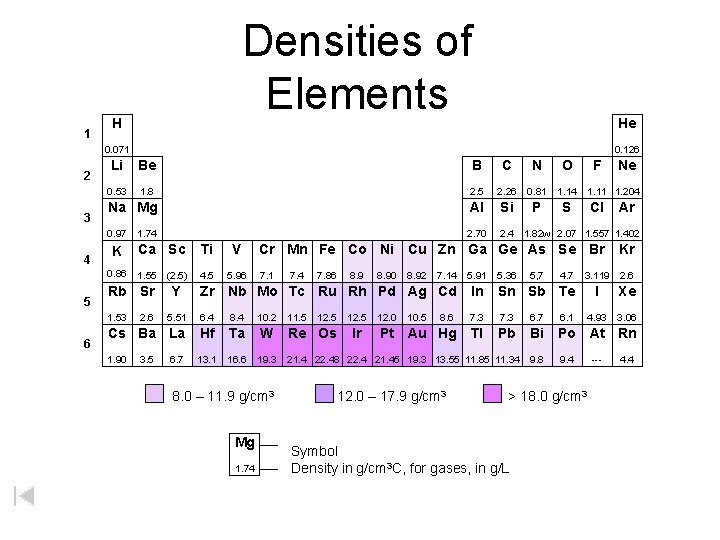
1 Densities of Elements H He 0. 071 2 3 4 5 Li Be B C N O 0. 53 1. 8 2. 5 2. 26 0. 81 1. 14 Na Mg Al Si P S 0. 97 2. 70 2. 4 1. 82 w 2. 07 1. 557 1. 402 1. 74 K Ca Sc Ti V 0. 86 1. 55 (2. 5) 4. 5 5. 96 Rb Sr Ne 1. 11 1. 204 Cl Ar Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 7. 1 3. 119 2. 6 I Xe 4. 93 3. 06 7. 4 7. 86 8. 90 8. 92 7. 14 5. 91 5. 36 5, 7 4. 7 In Sn Sb Te 5. 51 6. 4 8. 4 10. 2 8. 6 7. 3 6. 7 6. 1 Cs Ba La Hf Ta W Pt Au Hg Tl Pb Bi Po At Rn 1. 90 13. 1 16. 6 19. 3 9. 8 9. 4 2. 6 3. 5 Y F Zr Nb Mo Tc Ru Rh Pd Ag Cd 1. 53 6 0. 126 6. 7 8. 0 – 11. 9 g/cm 3 Mg 1. 74 W 11. 5 12. 5 Re Os 12. 5 Ir 12. 0 10. 5 21. 4 22. 48 22. 4 21. 45 19. 3 13. 55 11. 85 11. 34 12. 0 – 17. 9 g/cm 3 > 18. 0 g/cm 3 Symbol Density in g/cm 3 C, for gases, in g/L --- 4. 4
 Trend linier adalah
Trend linier adalah Metode trend non linear (kuadratis)
Metode trend non linear (kuadratis) What is periodicity?
What is periodicity? First dental home certification
First dental home certification Ikan euryphagic
Ikan euryphagic Chemsheets periodicity
Chemsheets periodicity Electronic configurations
Electronic configurations 42 electron configuration
42 electron configuration Ap chemistry chapter 7
Ap chemistry chapter 7 Bright futures screening guidelines
Bright futures screening guidelines Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Ionization energy on periodic table
Ionization energy on periodic table Life cycle of wuchereria bancrofti
Life cycle of wuchereria bancrofti Chapter 7 atomic structure and periodicity
Chapter 7 atomic structure and periodicity Chapter 9 section 1 labor market trends
Chapter 9 section 1 labor market trends In case of half sectioning, of the object is removed.
In case of half sectioning, of the object is removed. What is removed section
What is removed section Full section views
Full section views Describing energy section 2 answers
Describing energy section 2 answers Chapter 10 meiosis 1 and meiosis 2
Chapter 10 meiosis 1 and meiosis 2 Equifax national consumer credit trends report
Equifax national consumer credit trends report Ontogenetic market forecast
Ontogenetic market forecast Ionization energy trend
Ionization energy trend Enterprise search trends
Enterprise search trends Pre romantic poem
Pre romantic poem Packaging trends in india
Packaging trends in india Memory hierarchy
Memory hierarchy Emerging trends in community development
Emerging trends in community development Ionic radius trend
Ionic radius trend Recent trends in ic engine
Recent trends in ic engine Recent trends in project management
Recent trends in project management Legal implications in nursing practice
Legal implications in nursing practice Nystagmus market trends
Nystagmus market trends Progressivism educational philosophy
Progressivism educational philosophy Ion size trend
Ion size trend Atomic radius trend in periodic table
Atomic radius trend in periodic table Periodic trends
Periodic trends Periodic table of elements cheat sheet
Periodic table of elements cheat sheet Trends in pediatric nursing definition
Trends in pediatric nursing definition How does lattice energy increase on the periodic table
How does lattice energy increase on the periodic table Trends and issues in nursing
Trends and issues in nursing