periodic trends Periodic Trends properties that show patterns











- Slides: 27

periodic trends

Periodic Trends: properties that show patterns when examined across the periods or vertically down the groups • While there are many periodic trends, we focus on 1. oxidation numbers 2. atomic radius 3. ionic size 4. ionization energy 5. electronegativity 6. Reactivity

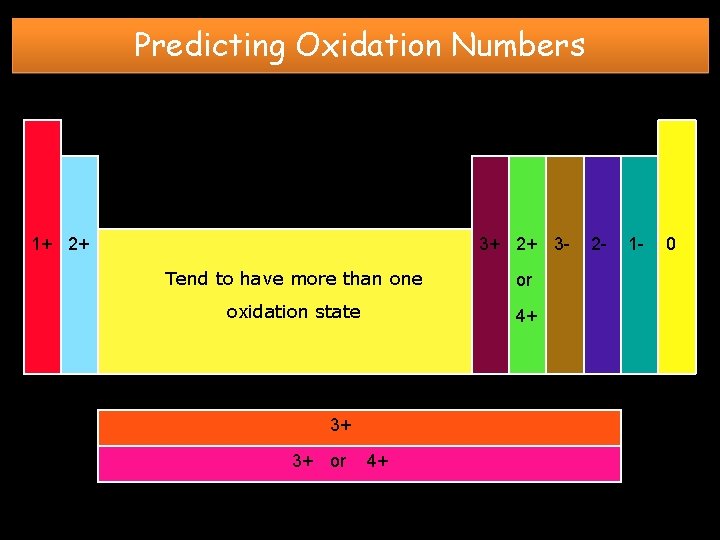
Predicting Oxidation Numbers • When an atom forms an ion, the stability of an octet of e- enables you to predict the number of eto be gained or lost. You can then predict the charge on the ion formed. • Oxidation Number - the electrical charge resulting from atoms gaining or losing electrons to fill their outermost s and p orbitals – Metals have (+) oxidation numbers (cations) – Nonmetals have (–) oxidation numbers (anions) – All Noble Gases have an oxidation number of zero (0)

Predicting Oxidation Numbers 1+ 2+ 3 Tend to have more than one or oxidation state 4+ 3+ 3+ or 4+ 2 - 1 - 0

Why trends? • Atomic structure and Coulombic attractions are responsible for the patterns in the following periodic properties • Specifically : – The attraction between protons in nucleus and valence electrons (nuclear charge) – The repulsion between electrons and other electrons in the electron cloud (shielding effect)

Nuclear charge • Nuclear charge – the net positive charge experienced by an electron from the attractive force of the proton in nucleus • Nuclear charge increases across a period – The number of protons increases but the energy level and number of core (inner) electrons stays the same

Nuclear charge PRACTICE: From each of the following pairs of atoms, select the atom that feels a GREATER force from the nucleus. 1. Na, Cl Cl – more nuclear charge 2. Se, Ca Se-more nuclear charge

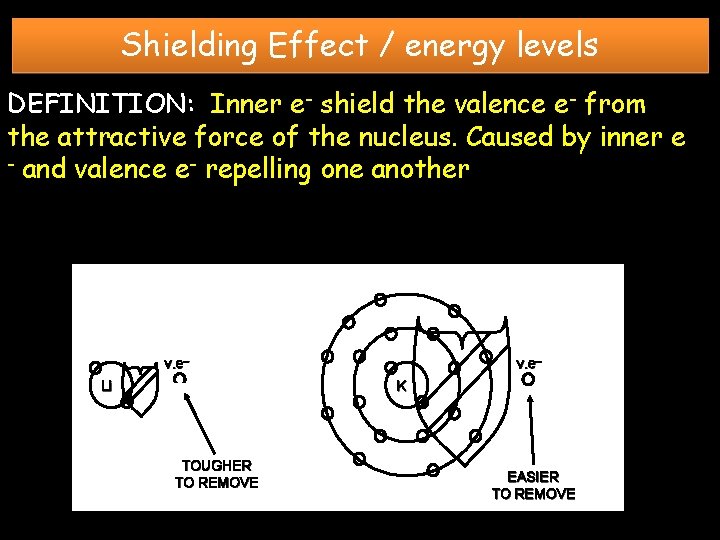
Shielding Effect / energy levels DEFINITION: Inner e- shield the valence e- from the attractive force of the nucleus. Caused by inner e - and valence e- repelling one another v. e– Li v. e– K TOUGHER TO REMOVE EASIER TO REMOVE

Shielding Effect GROUP TREND: TOP to BOTTOM INCREASES • New energy levels filled with electrons that shield the nucleus PERIODIC TREND: LEFT to RIGHT DOES NOT CHANGE • No change in the number of energy levels

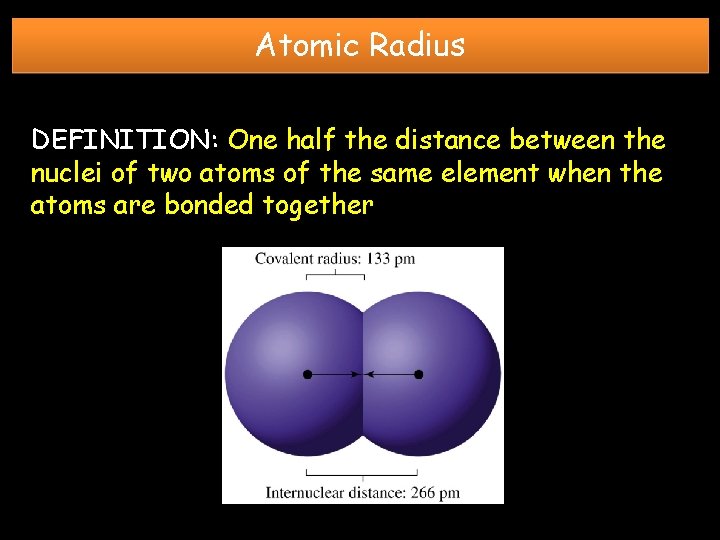
Atomic Radius DEFINITION: One half the distance between the nuclei of two atoms of the same element when the atoms are bonded together

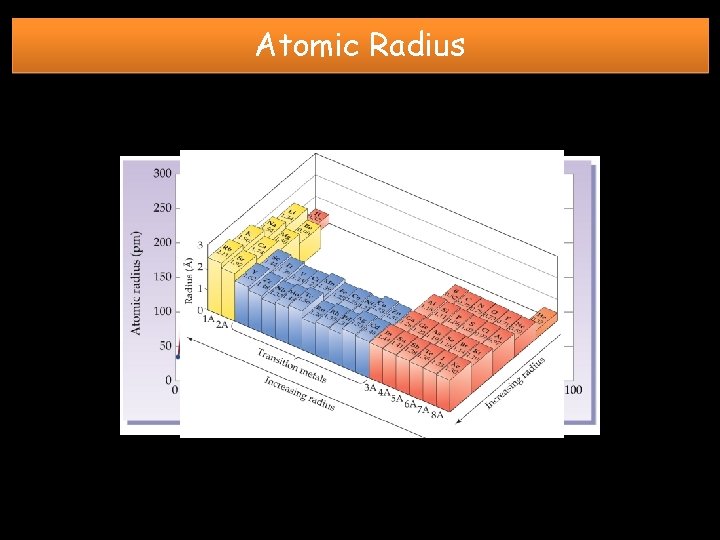
Atomic Radius GROUP TREND: TOP to BOTTOM INCREASES • New energy levels are added so the size of the electron cloud increases. PERIODIC TREND: LEFT to RIGHT DECREASES SLIGHTLY • No new energy levels are added • the “nuclear charge” increases by one proton for each element in the period. This pulls the electron cloud in a little tighter.

Atomic Radius

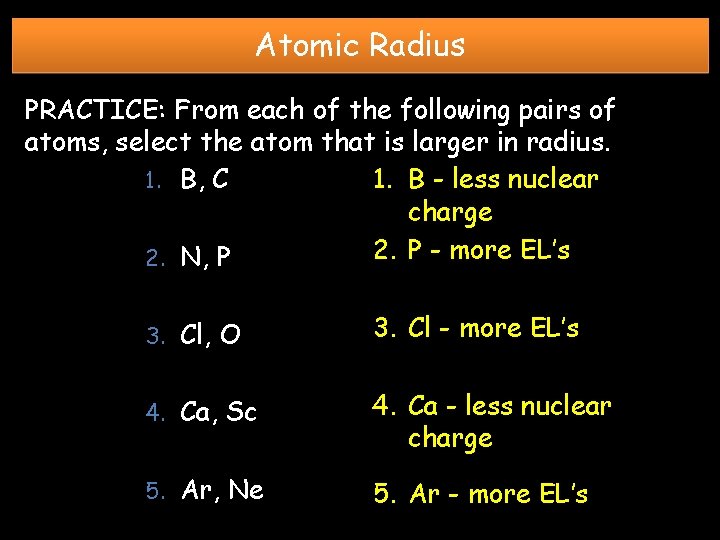
Atomic Radius PRACTICE: From each of the following pairs of atoms, select the atom that is larger in radius. 1. B, C 1. B - less nuclear charge 2. P - more EL’s 2. N, P 3. Cl, O 3. Cl - more EL’s 4. Ca, Sc 4. Ca - less nuclear charge 5. Ar, Ne 5. Ar - more EL’s

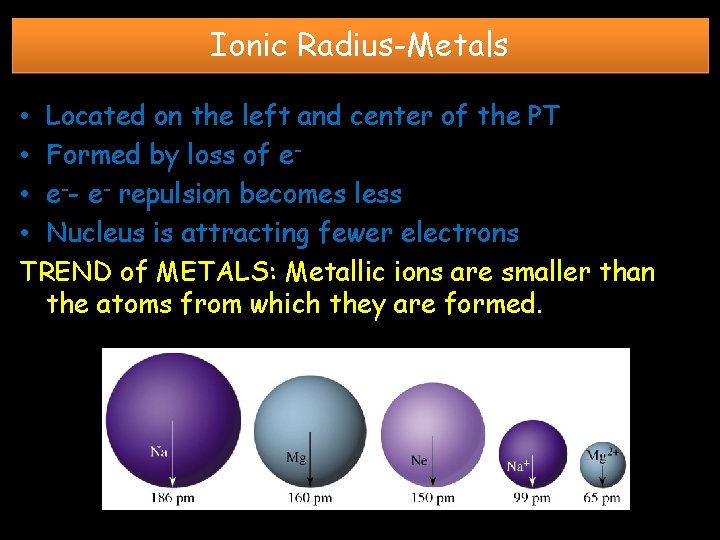
Ionic Radius-Metals • Located on the left and center of the PT • Formed by loss of e • e-- e- repulsion becomes less • Nucleus is attracting fewer electrons TREND of METALS: Metallic ions are smaller than the atoms from which they are formed.

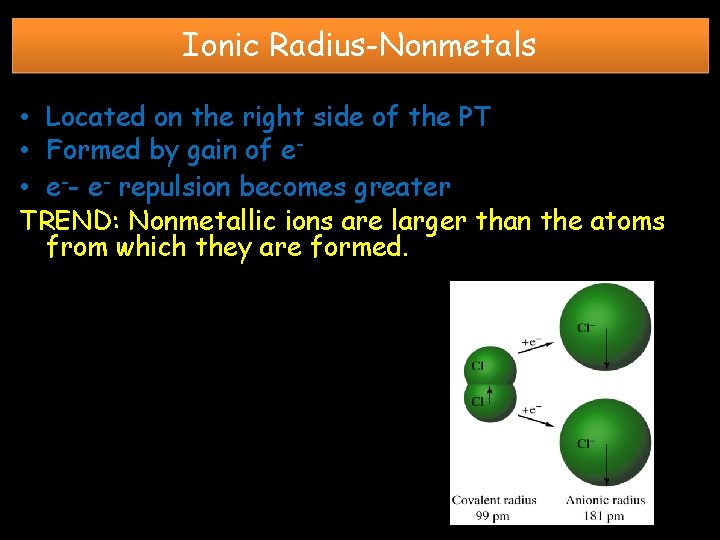
Ionic Radius-Nonmetals • Located on the right side of the PT • Formed by gain of e • e-- e- repulsion becomes greater TREND: Nonmetallic ions are larger than the atoms from which they are formed.

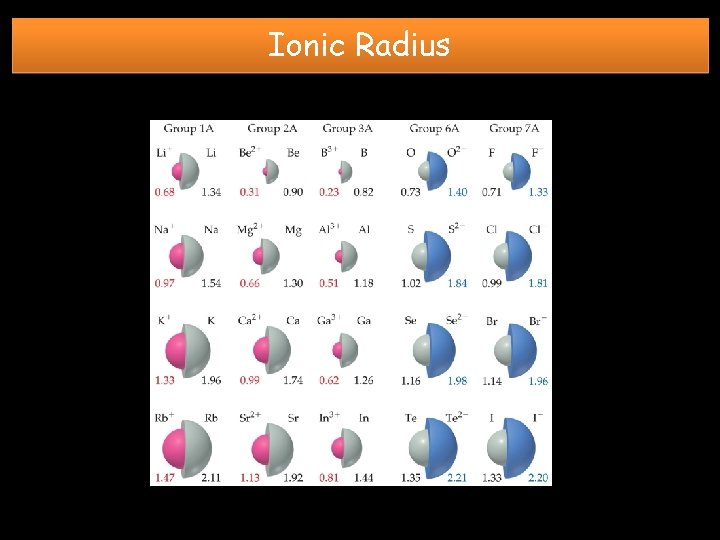
Ionic Radius

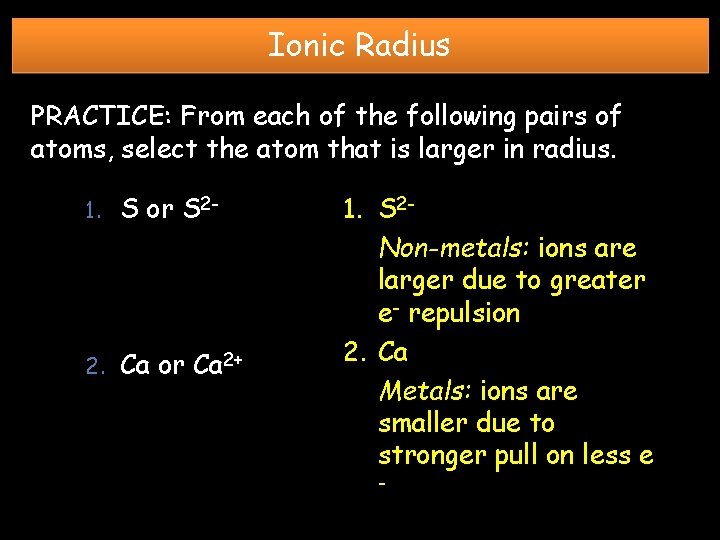
Ionic Radius PRACTICE: From each of the following pairs of atoms, select the atom that is larger in radius. 1. S or S 2 - 2. Ca or Ca 2+ 1. S 2 Non-metals: ions are larger due to greater e- repulsion 2. Ca Metals: ions are smaller due to stronger pull on less e -

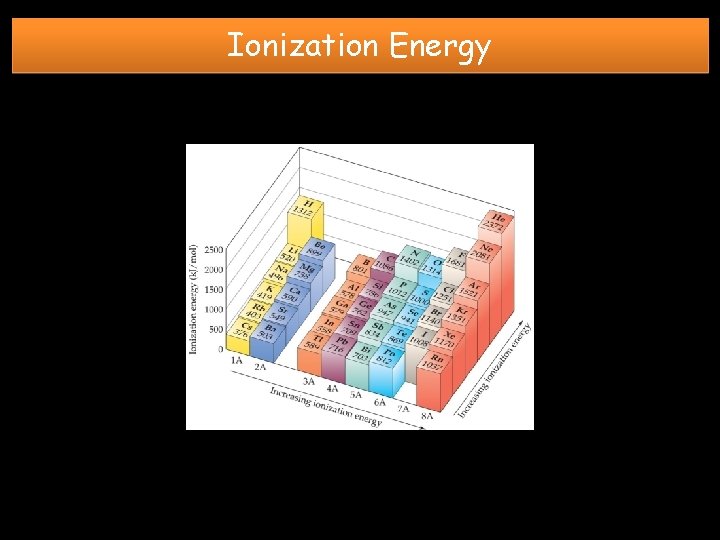
Ionization Energy DEFINITION: the energy required to remove the highest energy e- from a neutral atom GROUP TREND: TOP to BOTTOM DECREASES • Shielding effect and distance of outermost electrons from the nucleus increases due to addition of energy levels. Less energy is required to remove e-. PERIODIC TREND: LEFT to RIGHT INCREASES • Shielding effect is constant, but nuclear charge increases. More energy is required to remove e-.

Ionization Energy

Ionization Energy PRACTICE: From each of the following pairs of atoms, select the atom that has a lower first ionization energy. 1. Be, Mg 1. Mg-greater shielding, larger radius 2. Al, P 2. Al-smaller nuclear charge

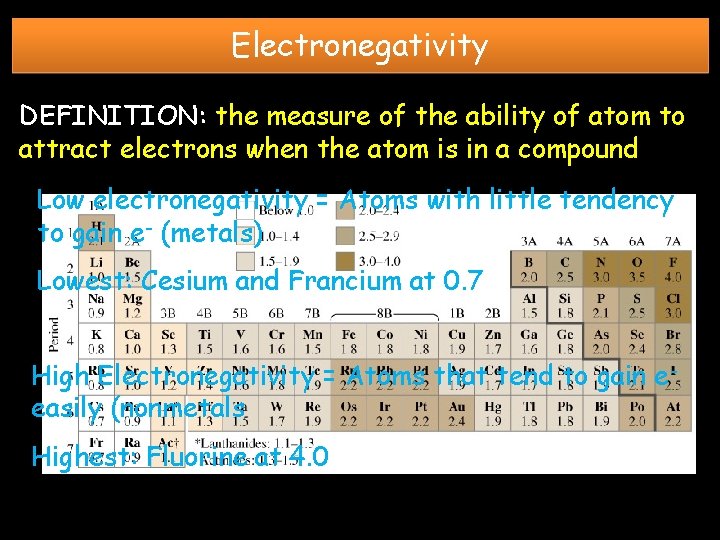
Electronegativity DEFINITION: the measure of the ability of atom to attract electrons when the atom is in a compound Low electronegativity = Atoms with little tendency to gain e- (metals) Lowest: Cesium and Francium at 0. 7 High Electronegativity = Atoms that tend to gain eeasily (nonmetals Highest: Fluorine at 4. 0

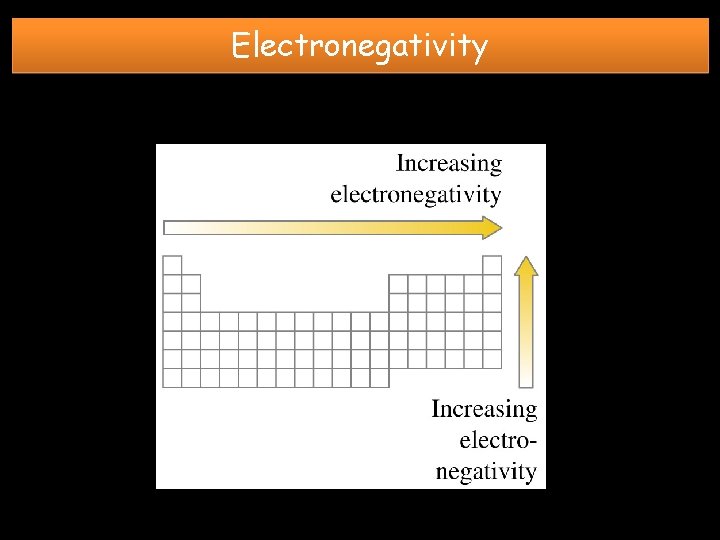
Electronegativity GROUP TREND: TOP to BOTTOM DECREASES • Shielding effect and distance of outermost electrons from the nucleus increases due to addition of energy levels. Less attraction for e-. PERIODIC TREND: LEFT to RIGHT INCREASES • Shielding effect is constant, but nuclear charge increases. More attraction for e-.

Electronegativity

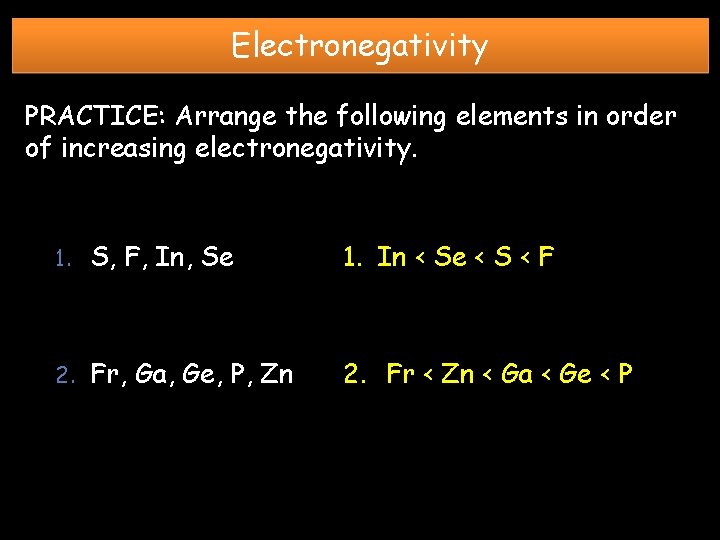
Electronegativity PRACTICE: Arrange the following elements in order of increasing electronegativity. 1. S, F, In, Se 1. In < Se < S < F 2. Fr, Ga, Ge, P, Zn 2. Fr < Zn < Ga < Ge < P

Reactivity of Metals GROUP TREND: TOP to BOTTOM MORE REACTIVE • A “larger” atom (more EL’s) will have less attraction for e- due to increased shielding • e- are more easily “lost” (i. e. , less pull from nucleus) from larger atom metals

Reactivity of Nonmetals GROUP TREND: TOP to BOTTOM LESS REACTIVE • A “smaller” atom (fewer EL’s) will have greater attraction for e- due to less shielding • e- are more easily “gained” (i. e. , more pull from nucleus) from smaller atom nonmetals

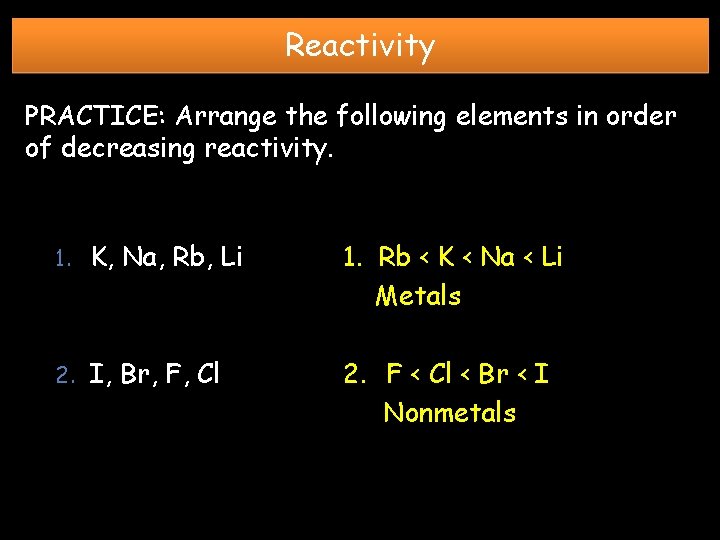
Reactivity PRACTICE: Arrange the following elements in order of decreasing reactivity. 1. K, Na, Rb, Li 1. Rb < K < Na < Li Metals 2. I, Br, F, Cl 2. F < Cl < Br < I Nonmetals