Periodic Properties PERIODIC PROPERTIES An elements properties can








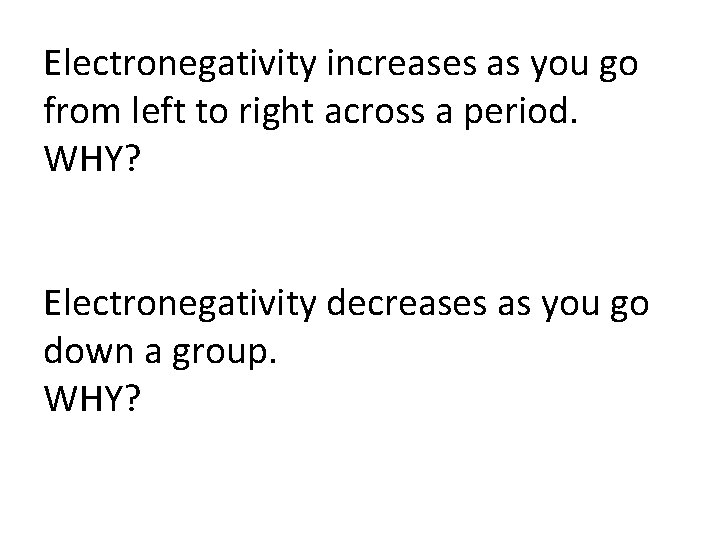








- Slides: 24

Periodic Properties PERIODIC PROPERTIES • An element’s properties can go hand in hand with electron arrangement • We can use an element’s location on the PT to predict many properties. – Atomic radius – Electron affinity – Electronegativity – Ionization energy – Ionic Size

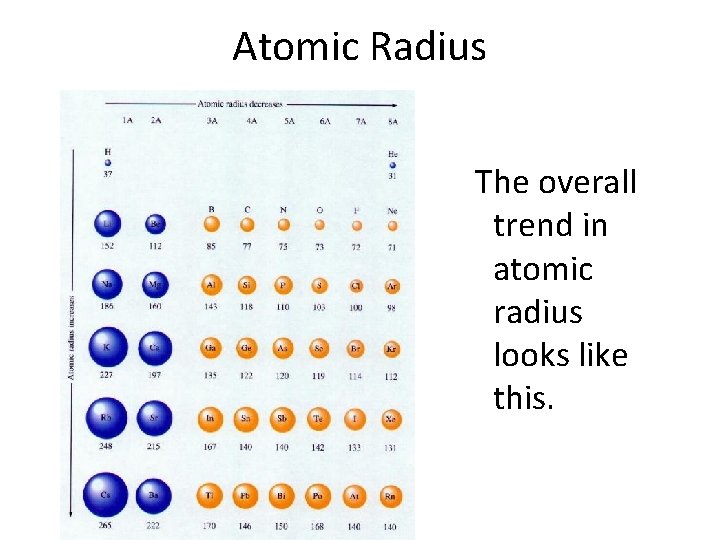
Atomic Radius • The trend for atomic radius in a vertical column is to go from smaller atoms at the top to larger atoms at the bottom of the family. • Why? • With each step down the family, a new energy level is added to the electron cloud, making the atoms larger with each step.

Atomic Radius • The trend across a horizontal period is less obvious. • What happens to atomic structure as we step from left to right? • Each element adds a proton and an electron (and 1 or 2 neutrons). • The electrons are added to existing energy levels or sublevels.

Atomic Radius • The effect is that the more positive nucleus has a greater pull on the electron cloud. • The nucleus is more positive and the electron cloud is about the same size. • The increased attraction pulls the cloud in, making atoms smaller as we move from left to right across a period.

Atomic Radius The overall trend in atomic radius looks like this.


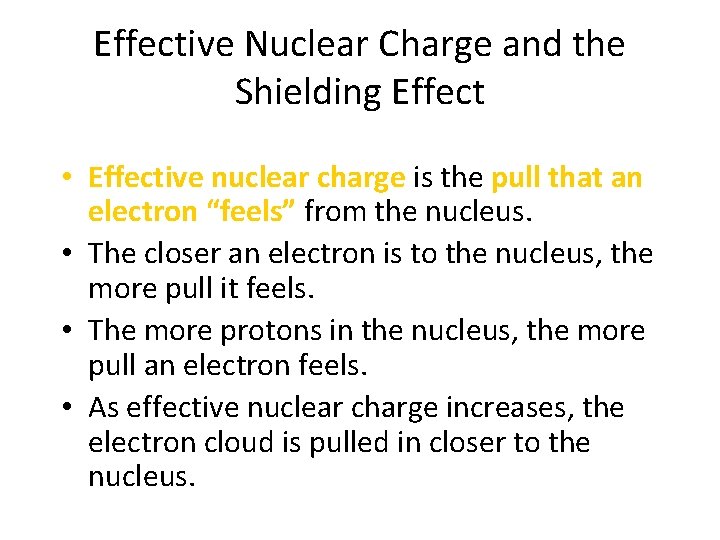
Effective Nuclear Charge and the Shielding Effect • Effective nuclear charge is the pull that an electron “feels” from the nucleus. • The closer an electron is to the nucleus, the more pull it feels. • The more protons in the nucleus, the more pull an electron feels. • As effective nuclear charge increases, the electron cloud is pulled in closer to the nucleus.

Effective Nuclear Charge and the Shielding Effect • Electrons in inner energy levels shield the outer electrons from the pull of the nucleus. • As you go from left to right across a period, the shielding effect does not change because the number of energy levels does not change (successive electrons are added to existing energy levels). • As you go down a group, the shielding effect increases with each additional energy level. • The shielding effect reduces the effective nuclear charge.

Electronegativity • Electronegativity (EN) is a measure of an atom’s attraction for the electrons in a covalent bond. • EN is measured with an arbitrary scale that ranges from 0 to 4. It is called the Pauling scale. There are no units. (EN first described by Linus Pauling. ) • Generally, metals are electron givers and have low electronegativities. • Nonmetals are electron takers and have high electronegativities. • F O Cl N Br I S C – a goofy way to remember the eight most electronegative elements

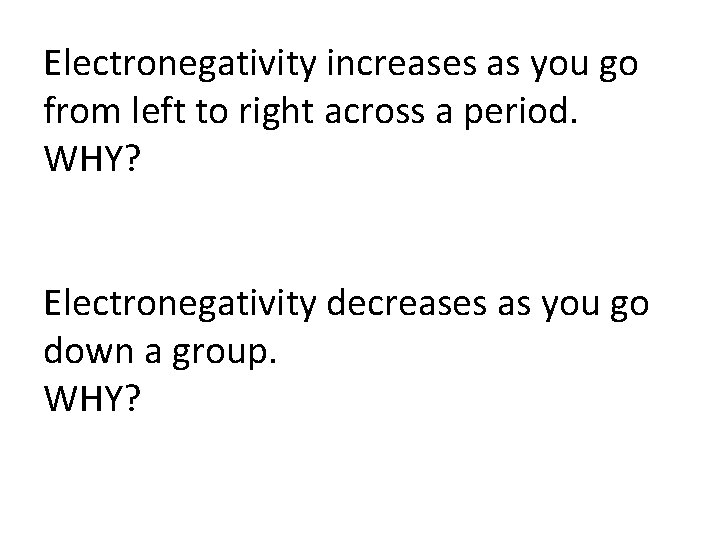
Electronegativity increases as you go from left to right across a period. WHY? Electronegativity decreases as you go down a group. WHY?

Ionization Energy • If an electron is given enough energy (in the form of a photon) to overcome the effective nuclear charge holding the electron in the cloud, it can leave the atom completely. • The atom has been “ionized” or charged. • The number of protons and electrons is no longer equal.

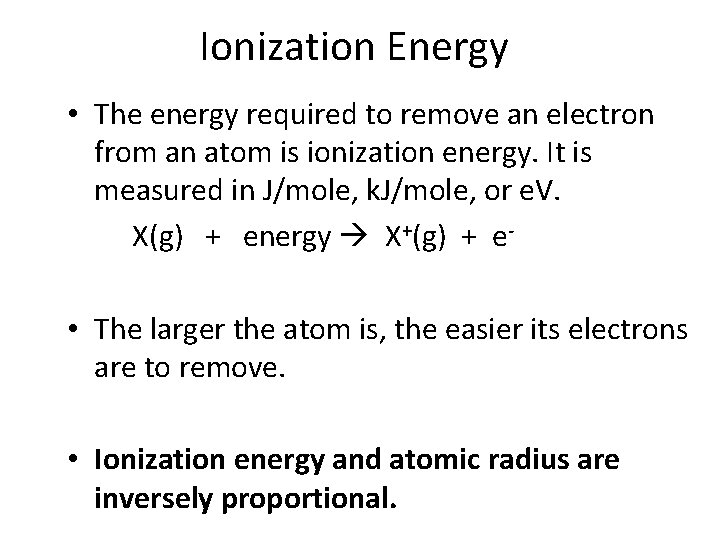
Ionization Energy • The energy required to remove an electron from an atom is ionization energy. It is measured in J/mole, k. J/mole, or e. V. X(g) + energy X+(g) + e • The larger the atom is, the easier its electrons are to remove. • Ionization energy and atomic radius are inversely proportional.

First Ionization Energies

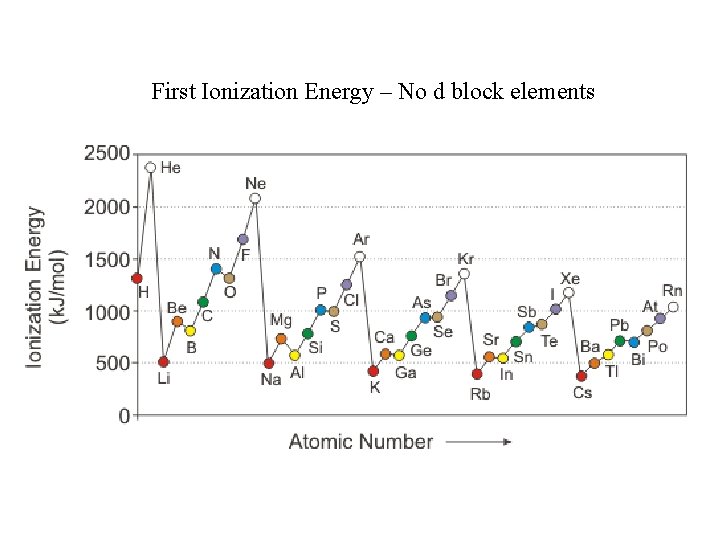
First Ionization Energy – No d block elements


Ionization energy increases as you go from left to right across a period. WHY? Ionization energy decreases as you go down a group. WHY?

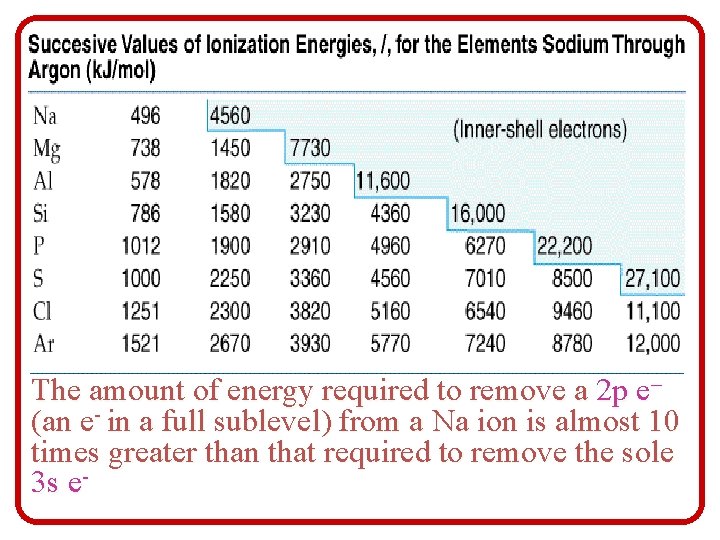
1 st IE – energy required to remove the 1 st (outermost) electron Al(g) + energy Al+(g) + e¯ 2 nd IE – energy required to remove the 2 nd electron Al+(g) + energy Al+2(g) + e¯ 3 rd IE – energy required to remove the 3 rd electron Al+2(g) + energy Al+3(g) + e¯

The amount of energy required to remove a 2 p e– (an e- in a full sublevel) from a Na ion is almost 10 times greater than that required to remove the sole 3 s e-

ELECTRON AFFINITY • Electron affinity is a measure of the ability of an atom to attract or gain an electron. • First electron affinity is the energy released when one mole of gaseous atoms each acquire an electron to form one mole of gaseous -1 ions. X(g) + e¯ X¯ + Energy k. J/mole X¯ is at a lower energy state than X The greater the effective nuclear charge, the higher the first electron affinity (the more energy is released).

• First electron affinities have negative values. • The negative sign indicates a release of energy. • Electron affinity is a measure of the attraction between the incoming e¯ and the nucleus. The stronger the attraction the more energy is released. • The factors that effect this attraction are nuclear charge, distance from the nucleus and shielding effect.


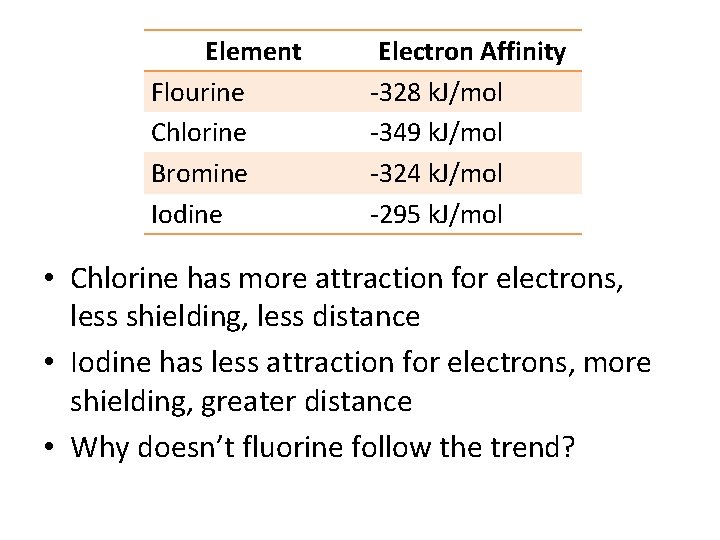
Element Flourine Chlorine Bromine Iodine Electron Affinity -328 k. J/mol -349 k. J/mol -324 k. J/mol -295 k. J/mol • Chlorine has more attraction for electrons, less shielding, less distance • Iodine has less attraction for electrons, more shielding, greater distance • Why doesn’t fluorine follow the trend?

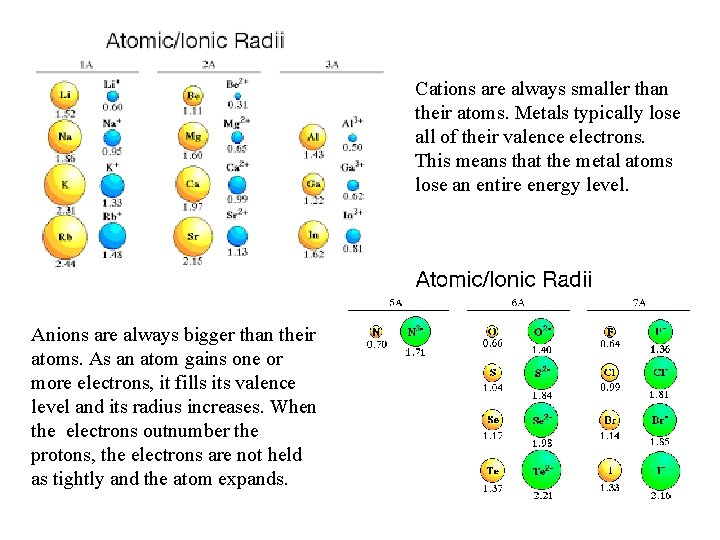
Cations are always smaller than their atoms. Metals typically lose all of their valence electrons. This means that the metal atoms lose an entire energy level. Anions are always bigger than their atoms. As an atom gains one or more electrons, it fills its valence level and its radius increases. When the electrons outnumber the protons, the electrons are not held as tightly and the atom expands.