PERIODIC TABLE PERIODIC TABLE The periodic table is

PERIODIC TABLE


PERIODIC TABLE • The periodic table is a chart of the elements arranged into rows and columns according to their physical and chemical properties
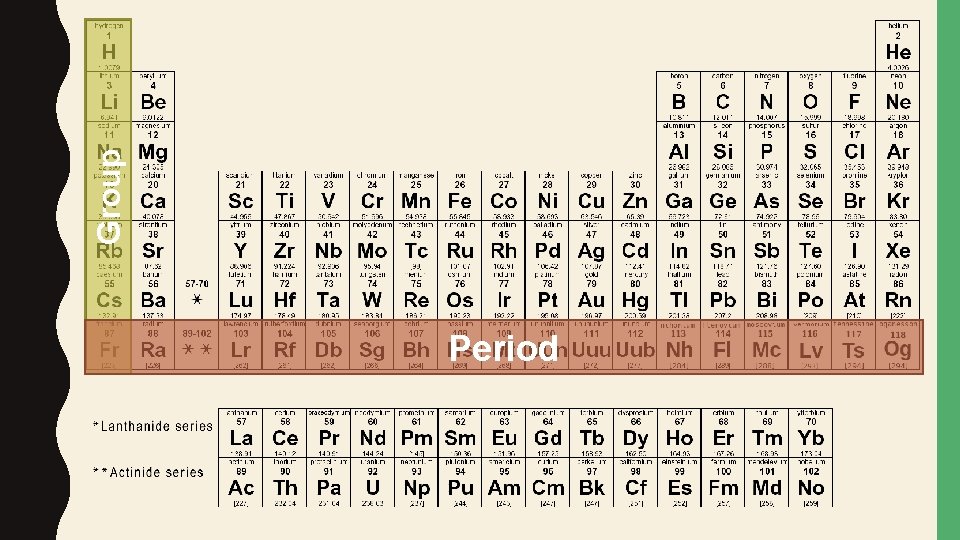
Group Period

GROUPS • A group is a column on the periodic table. Elements in the same group have similar chemical properties and react with other elements in similar ways
PERIODS • A period is a row on the periodic table. The physical and chemical properties change as you move across a period.

LESSON 2 METALS
METAL • A metal is an element that is generally shiny. It is easily pulled into wires or hammered into thin sheets. A metal is a good conductor of electricity and thermal energy

HTTPS: //WWW. BRAINPOP. COM/SCIENC E/MATTERANDCHEMISTRY/METALS/

PHYSICAL PROPERTIES OF METALS • Luster: ability of a metal to reflect light • Conductivity: ability to conduct electricity • Ductility: ability to be pulled into thin wires • Malleability: ability of a substance to be hammered or rolled into sheets

CHEMICAL PROPERTIES OF METALS • Ability to rust • Flammability • Reactivity with other substances

GROUP 1: ALKALI METALS • Examples: lithium, sodium, potassium • React quickly with other elements such as oxygen • Occur only in compounds in nature • Silvery appearance, soft enough to cut with a knife, lowest densities

GROUP 2: ALKALINE EARTH METALS • Examples: beryllium, magnesium, calcium • React quickly with other elements but not as quickly as the alkali metals • Low density but higher than alkali metals

GROUPS 3 -12: TRANSITION ELEMENTS • Examples: copper, silver, nickel, iron, and gold • All transition elements are metals • They have higher melting points, greater strength and higher densities than alkali metals and alkaline earth metals • Resist corrosion • They react less quickly with oxygen
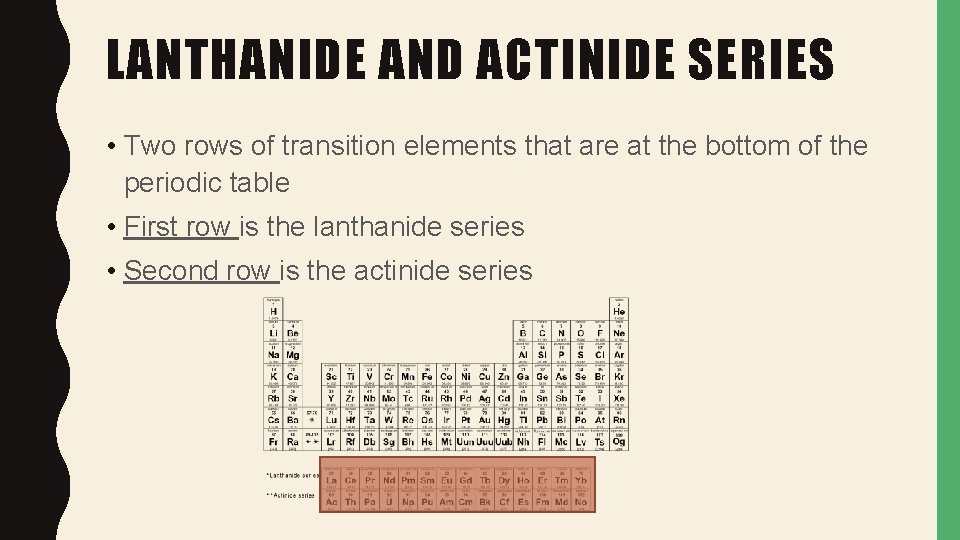
LANTHANIDE AND ACTINIDE SERIES • Two rows of transition elements that are at the bottom of the periodic table • First row is the lanthanide series • Second row is the actinide series

HOW ARE ELEMENTS GROUPED IN THE PERIODIC TABLE? Elements are grouped in the periodic table according to similarities of their properties.

LESSON 3 NONMETALS AND METALLOIDS

NONMETALS • Nonmetals are elements that have no metallic properties • Gases at room temperature • Located on the right side • Examples: nitrogen, oxygen, carbon, phosphorus and sulfur


HTTPS: //WWW. BRAINPOP. COM/SCIENC E/MATTERANDCHEMISTRY/PERIODICTAB LEOFELEMENTS/
PROPERTIES OF NONMETALS • Those that are solid at room temperature have a dull surface (no luster) • Poor conductors of electricity and thermal energy (good insulators)

GROUP 14 -16 NONMETALS • Carbon is the only nonmetal in group 14 • Nitrogen and phosphorus are the only nonmetals in group 15 • Oxygen, sulfur, and selenium are the only nonmetals in group 16

GROUP 17: THE HALOGENS • Examples: fluorine, chlorine, bromine, and iodine • Elements that can react with a metal to form a salt • Only occur naturally in compounds

GROUP 18: THE NOBLE GASES • Examples: helium, neon, argon, krypton, xenon, and radon • Do not form compounds except under special circumstances in a laboratory

HYDROGEN • Has the smallest atomic mass • Most common element in the universe • Classified as a nonmetal but conducts electricity in its liquid form and reacts like an alkali metal • On Earth, hydrogen behaves like a nonmetal

METALLOIDS • A metalloid is an element that has physical and chemical properties of both metals and nonmetals • Examples: boron, silicon, germanium, and arsenic

PROPERTIES OF METALLOIDS • Metalloids are semiconductors meaning they conduct electricity at high temperatures, but not at low temperatures • Useful for electronic devices such as computers, televisions, and solar cells

GROUP 1 AND 17 Most readily combine

SULFUR AND PHOSPHORUS Nonmetals

COPPER AND IRON Metals

GROUP 17 Halogens
- Slides: 32