PERIODIC TABLE STRUCTURE PERIODIC STRUCTURE 3 Main Periodic
PERIODIC TABLE STRUCTURE
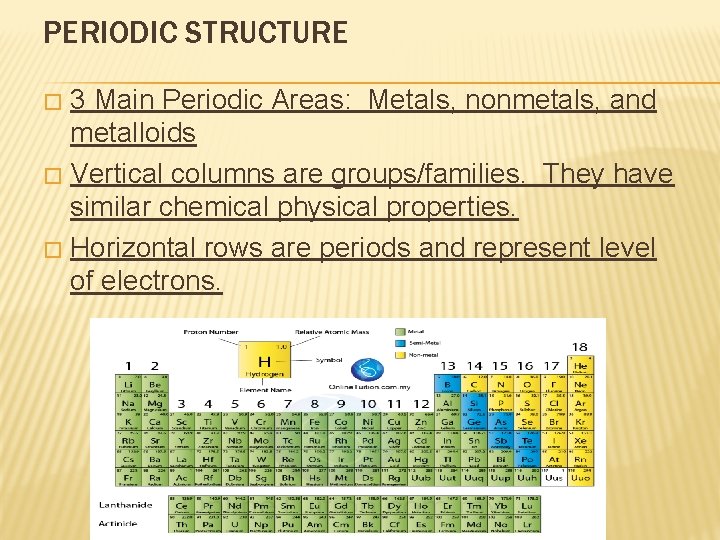
PERIODIC STRUCTURE 3 Main Periodic Areas: Metals, nonmetals, and metalloids � Vertical columns are groups/families. They have similar chemical physical properties. � Horizontal rows are periods and represent level of electrons. �
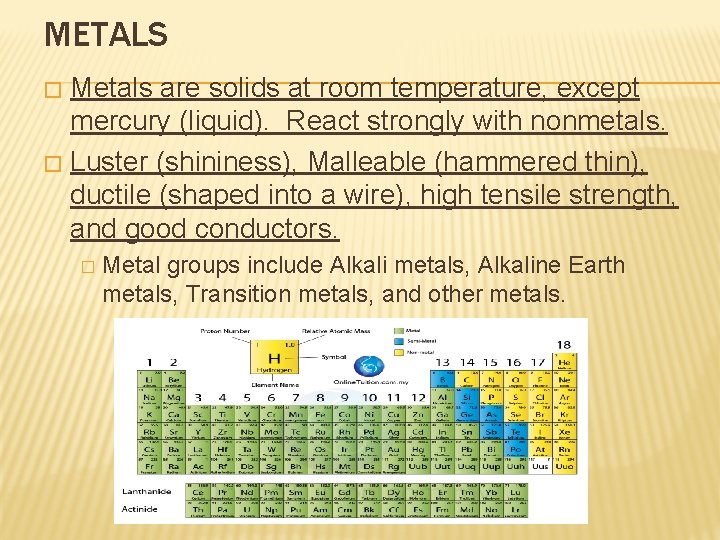
METALS Metals are solids at room temperature, except mercury (liquid). React strongly with nonmetals. � Luster (shininess), Malleable (hammered thin), ductile (shaped into a wire), high tensile strength, and good conductors. � � Metal groups include Alkali metals, Alkaline Earth metals, Transition metals, and other metals.
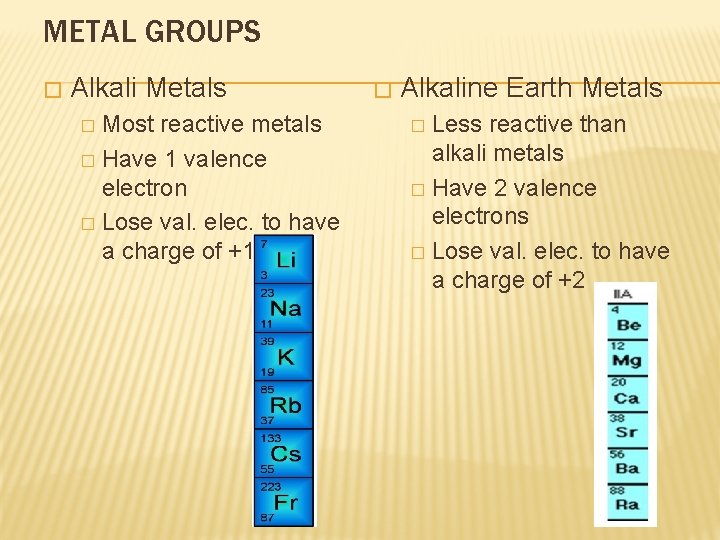
METAL GROUPS � Alkali Metals Most reactive metals � Have 1 valence electron � Lose val. elec. to have a charge of +1. � � Alkaline Earth Metals Less reactive than alkali metals � Have 2 valence electrons � Lose val. elec. to have a charge of +2 �
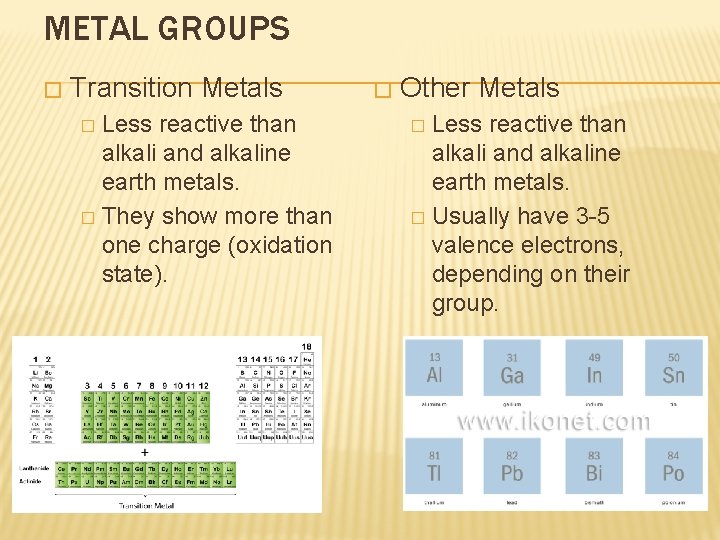
METAL GROUPS � Transition Metals Less reactive than alkali and alkaline earth metals. � They show more than one charge (oxidation state). � � Other Metals Less reactive than alkali and alkaline earth metals. � Usually have 3 -5 valence electrons, depending on their group. �
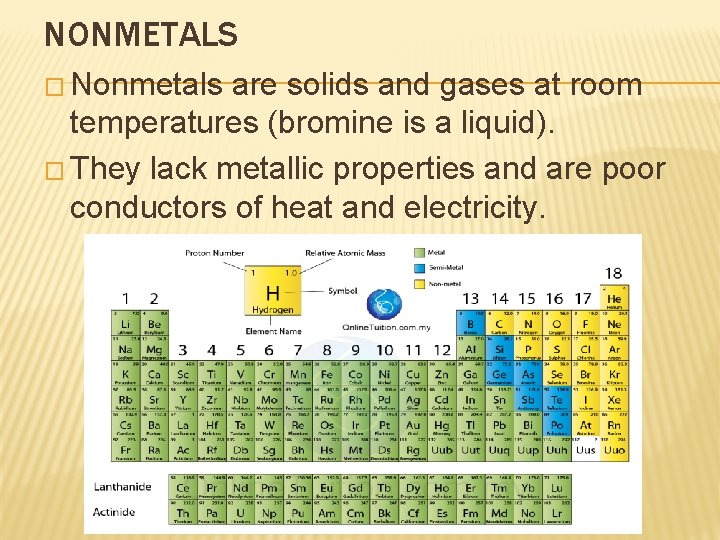
NONMETALS � Nonmetals are solids and gases at room temperatures (bromine is a liquid). � They lack metallic properties and are poor conductors of heat and electricity.

NONMETAL GROUPS � Halogens Most reactive nonmetals � Contain 7 valence electrons. � Gain a val. elec. to have a charge of -1. � � Noble Gases Least reactive group on the periodic table. � Full valence shells (2 or 8 depending on element). �
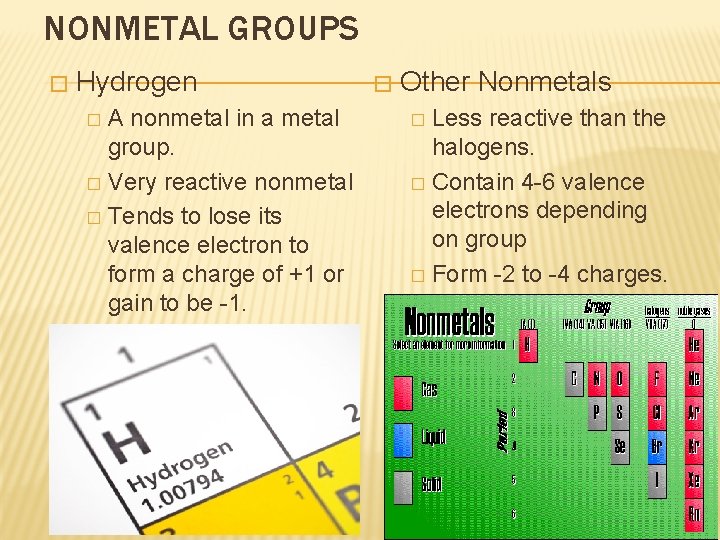
NONMETAL GROUPS � Hydrogen A nonmetal in a metal group. � Very reactive nonmetal � Tends to lose its valence electron to form a charge of +1 or gain to be -1. �. � � Other Nonmetals Less reactive than the halogens. � Contain 4 -6 valence electrons depending on group � Form -2 to -4 charges. �
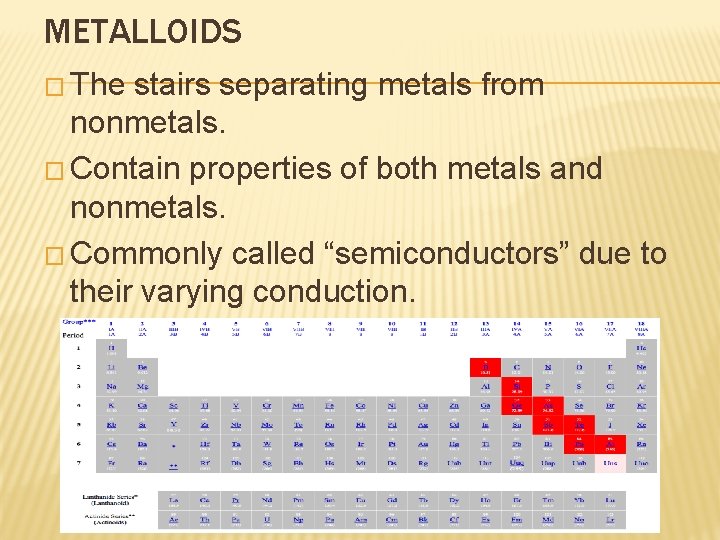
METALLOIDS � The stairs separating metals from nonmetals. � Contain properties of both metals and nonmetals. � Commonly called “semiconductors” due to their varying conduction.
- Slides: 9