Periodic Trends Types of Periodic Trends 1 Atomic



















- Slides: 29

Periodic Trends > Types of Periodic Trends 1. Atomic Radii (AR) 2. Ionization Energy (IE) 3. Electronegativity (EN) 4. Ionic Radii (IR) 5. Metallic Charateristic (MC) 6. Valence Electron (VE) 7. Meling Point (MP) © Copyright Pearson Prentice Hall Slide 1 of 31

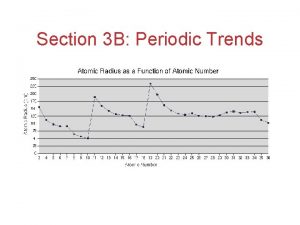
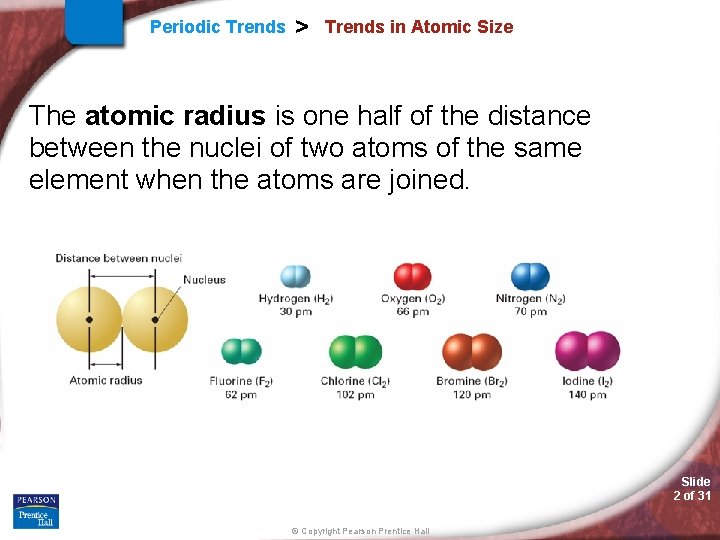
Periodic Trends > Trends in Atomic Size The atomic radius is one half of the distance between the nuclei of two atoms of the same element when the atoms are joined. Slide 2 of 31 © Copyright Pearson Prentice Hall

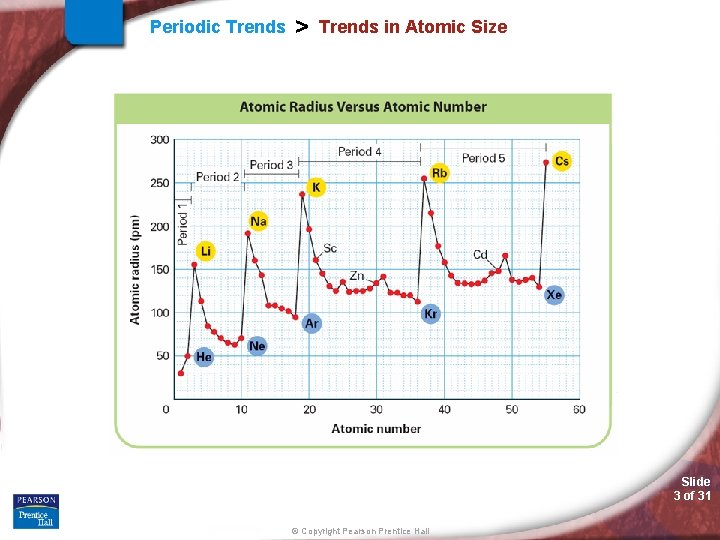
Periodic Trends > Trends in Atomic Size Slide 3 of 31 © Copyright Pearson Prentice Hall

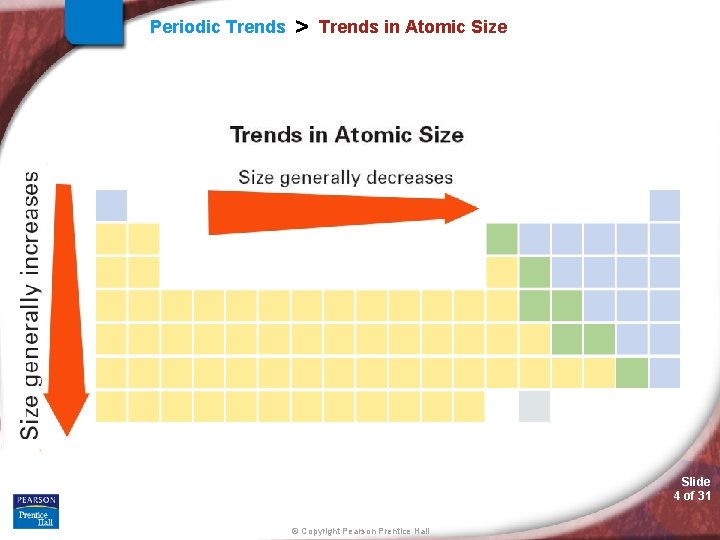
Periodic Trends > Trends in Atomic Size Slide 4 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Atomic Radii (AR) Periodic Trend for Atomic Radii (AR) Down a Group: Trend: Reason: Across a Period: Trend: Reason: Slide 5 of 31 © Copyright Pearson Prentice Hall

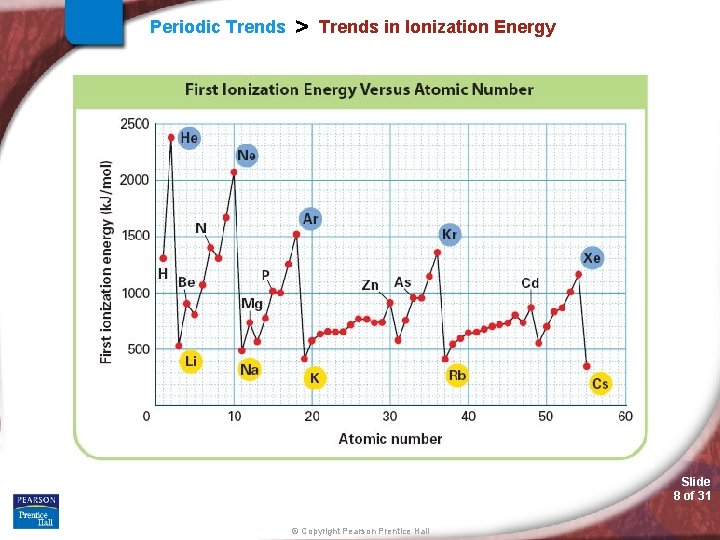
Periodic Trends > Trends in Ionization Energy (IE) - The energy required to remove the first valence electron from an atom (atom is in the gas phase). • What is holding the valence electron to the atom? • Valence electron tightly held or loosely? Slide 6 of 31 © Copyright Pearson Prentice Hall

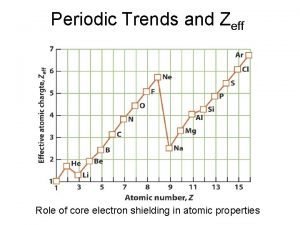
Periodic Trends > Shielding As more energy levels are added to atoms, the inner layers of electrons shield the outer electrons from the nucleus. The effective nuclear charge on those outer electrons is less, and so the outer electrons are less tightly held. Slide 7 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Trends in Ionization Energy Slide 8 of 31 © Copyright Pearson Prentice Hall

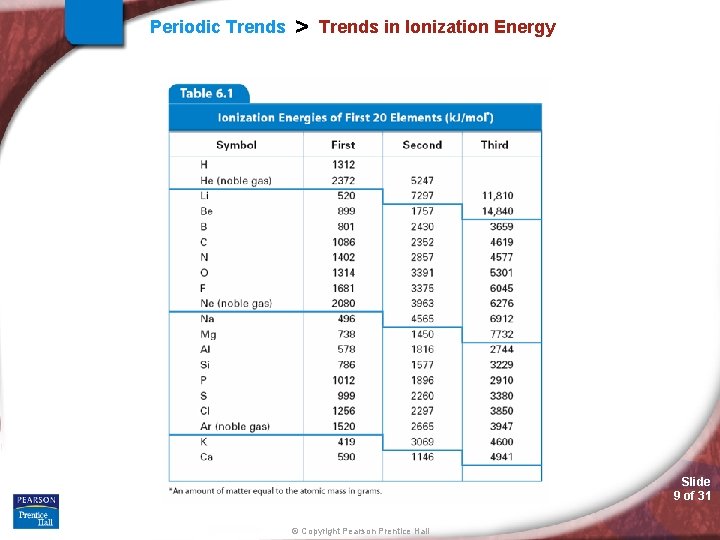
Periodic Trends > Trends in Ionization Energy Slide 9 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Trends in Ionization Energy Slide 10 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Ionization Energy (IE) Periodic Trend for Ionization Energy (IE) Down a Group: Trend: Reason: Across a Period: Trend: Reason: Slide 11 of 31 © Copyright Pearson Prentice Hall

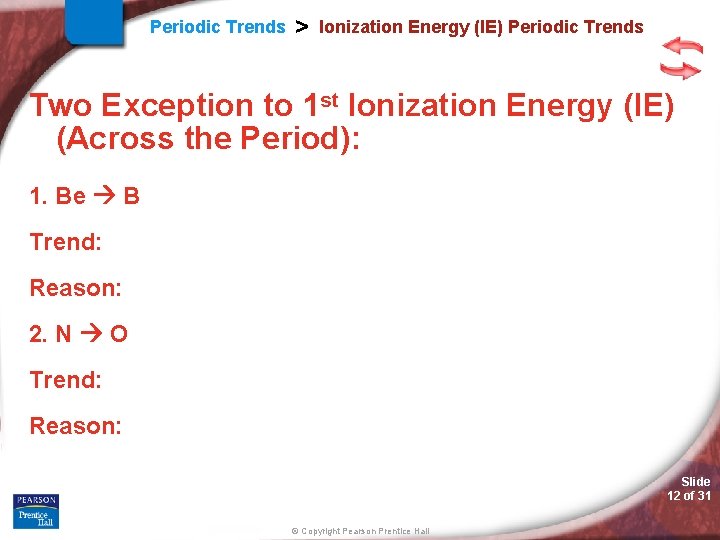
Periodic Trends > Ionization Energy (IE) Periodic Trends Two Exception to 1 st Ionization Energy (IE) (Across the Period): 1. Be B Trend: Reason: 2. N O Trend: Reason: Slide 12 of 31 © Copyright Pearson Prentice Hall

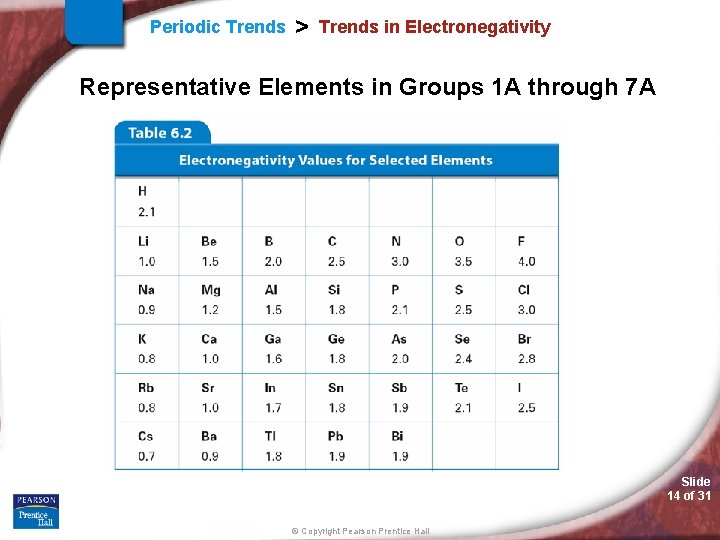
Periodic Trends > Trends in Electronegativity • Trends in Electronegativity is the ability of an atom, in a chemical bond, to attract the shared valence electrons to itself. (i. e. the shared valence electrons are physically closer to the higher EN value atom than the other atom in the chemical bond). • What EN really means? • Fluorine? Slide 13 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Trends in Electronegativity Representative Elements in Groups 1 A through 7 A Slide 14 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Electronegativity (EN) Periodic Trend for Electronegativity (EN) Down a Group: Trend: Reason: Across a Period: Trend: Reason: Slide 15 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Ions An ion is an atom or group of atoms that has a positive or negative charge. • A cation is an ion with a positive charge. • An anion is an ion with a negative charge. Slide 16 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Ions Metals elements lose valence electrons to form cation ions. Cation radii (IR) are always smaller than atomic radii (AR) [i. e. IR < AR] Slide 17 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Ions Non-metal elements gain valence electrons to form anion ions. Anion radii (IR) are always larger than atomic radii (AR) [i. e. IR > AR] Slide 18 of 31 © Copyright Pearson Prentice Hall

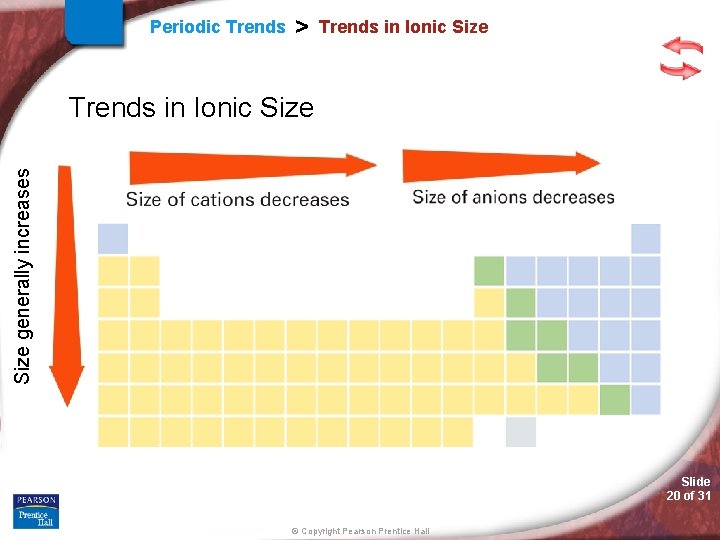
Periodic Trends > Trends in Ionic Size Relative Sizes of Some Atoms and Ions Slide 19 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Trends in Ionic Size generally increases Trends in Ionic Size Slide 20 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Ionic Radii Periodic Trend for Ionic Radii (IR) Down a Group: Trend: Reason: Across a Period: Metals large small, then Non-metal large small (Caution: Are not looking at same charge ion when across period) Metals: IR vs. AR : Reason: Non-metals: IR vs. AR : Reason: Slide 21 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Definition of Metals Composed of cations in a “sea” of free flowing valence electrons. Metals are lustrous (shiny), malleable, ductile, and are good conductors of heat and electricity. They are mostly solids at room temp. What is one exception? Slide 22 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Nonmetals are the opposite. They are dull, brittle, nonconductors (insulators). Some are solid, but many are gases, and Bromine is a liquid. Slide 23 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Metalloids, aka semi-metals are just that. They have characteristics of both metals and nonmetals. They are shiny but brittle. And they are semiconductors. What is our most important semiconductor? Slide 24 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Metallic Character This is simply a relative measure of how easily atoms lose or give up electrons. Slide 25 of 31 © Copyright Pearson Prentice Hall

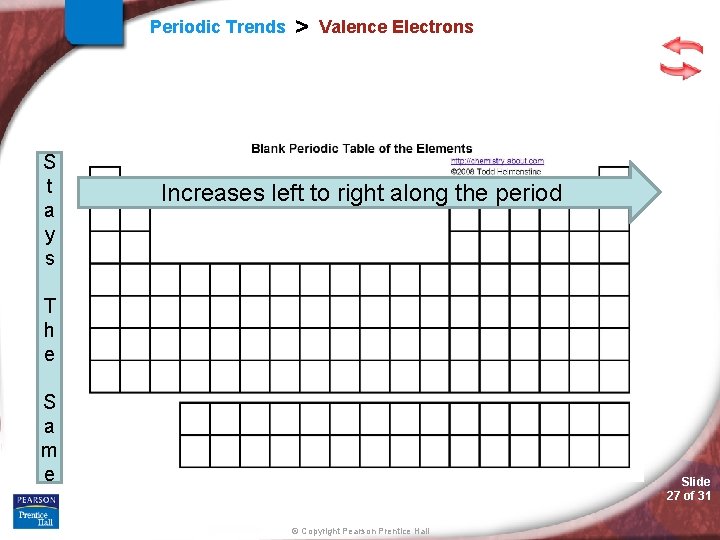
Periodic Trends > Valence Electrons Electron configuration Elements within a group end with the same sublevel and # of electrons within that sublevel s-block, p-block, d-block, f-block Slide 26 of 31 © Copyright Pearson Prentice Hall

Periodic Trends S t a y s > Valence Electrons Increases left to right along the period T h e S a m e Slide 27 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > The temperature at which a solid becomes a liquid. Bonds break between atoms/molecules. Metals generally possess a high melting point. Most non-metals possess low melting points. Trend splits Metals, increases left to right Nonmetal, decreases left to right Slide 28 of 31 © Copyright Pearson Prentice Hall

Periodic Trends > Nonmetals Increase Right to Left Metals increase Left to Right I n c r e a s e s U p Slide 29 of 31 © Copyright Pearson Prentice Hall
 Small atomic radius
Small atomic radius Is sulfer a cation or anion
Is sulfer a cation or anion Periyodik tablo periodic trends
Periyodik tablo periodic trends How to find out group and period of an element
How to find out group and period of an element Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Atomic mass of oxygen
Atomic mass of oxygen Distinguish between mass number and atomic mass.
Distinguish between mass number and atomic mass. Atomic number vs atomic radius
Atomic number vs atomic radius Atomic mass periodic trend
Atomic mass periodic trend Trends of ionization energy
Trends of ionization energy Atom size and electronegativity
Atom size and electronegativity Periodic table with atomic mass
Periodic table with atomic mass Atomic emission spectra periodic table
Atomic emission spectra periodic table Structure of atom periodic table
Structure of atom periodic table Atomic radius
Atomic radius Increasing atomic size periodic table
Increasing atomic size periodic table Mendeleev
Mendeleev Periodic table basics
Periodic table basics Ap chemistry chapter 7 atomic structure and periodicity
Ap chemistry chapter 7 atomic structure and periodicity Graphing periodic trends
Graphing periodic trends Oxidation trends periodic table
Oxidation trends periodic table Electronegativity meaning
Electronegativity meaning Periodic trend zeff
Periodic trend zeff Periodic trends activity worksheet
Periodic trends activity worksheet Periodic trends practice questions
Periodic trends practice questions Periodic trends in reactivity
Periodic trends in reactivity Summary of periodic trends
Summary of periodic trends Periodic trends practice problems
Periodic trends practice problems Coulomb's law periodic trends
Coulomb's law periodic trends Periodic trends electron affinity
Periodic trends electron affinity