Relative atomic and molecular mass Including introduction to








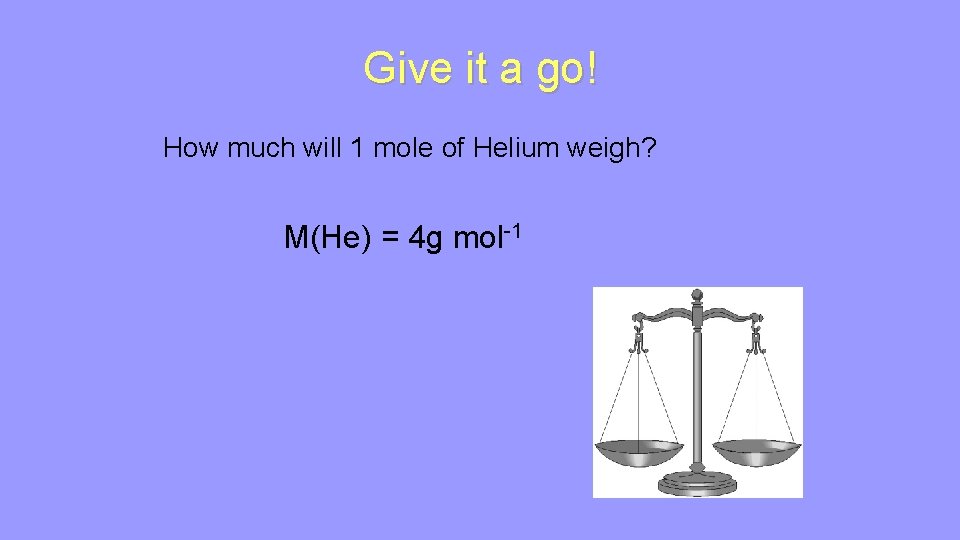


- Slides: 23

Relative atomic and molecular mass Including introduction to the mole

Objectives: • Recap of the structure of the atom • To understand the need for relative atomic mass • How to find relative atomic mass on the periodic table • To know how to calculate relative formula (molecular) mass • Checking balanced equations with relative formula masses • Definition of the mole • Calculation of molecular masses

Mass number tells us how many protons and neutrons are in the nucleus 23 Proton number tells us how many protons are in the nucleus Na 11 What does this tell us? Recap… Mass of a proton? Neutron? Electron?

Atomic Mass Units The actual mass of a hydrogen atom is 1. 7 x 10 -24 g (that’s 0. 00000000000017 g!) Far too small a number to easily get your head around… So – we use Relative Atomic Masses.

Relative Atomic Mass (Ar) tells us the mass of an atom compared to 1 atom of carbon. Carbon is given a Ar of 12 (as it has 6 protons and 6 neutrons).

What is relative atomic mass? 6 of 34 © Boardworks Ltd 2011

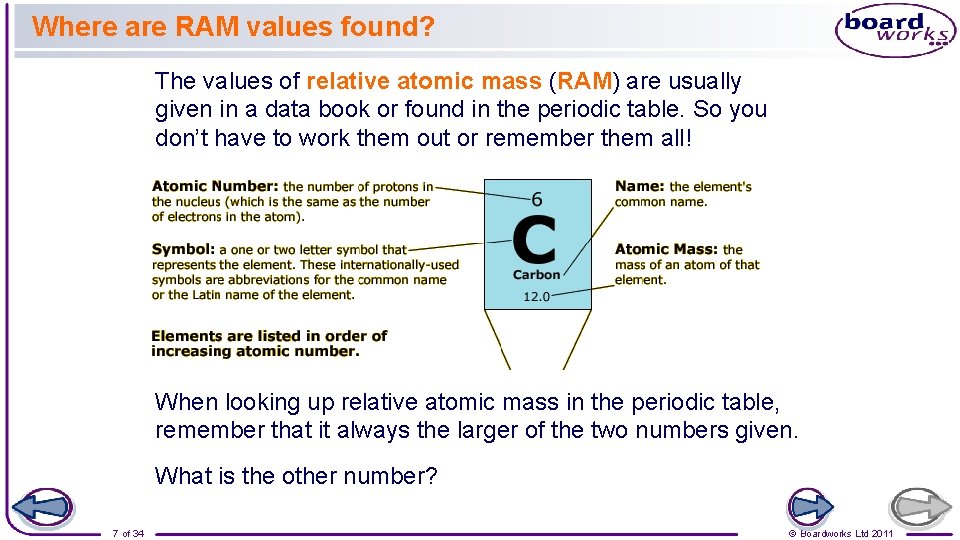
Where are RAM values found? The values of relative atomic mass (RAM) are usually given in a data book or found in the periodic table. So you don’t have to work them out or remember them all! When looking up relative atomic mass in the periodic table, remember that it always the larger of the two numbers given. What is the other number? 7 of 34 © Boardworks Ltd 2011

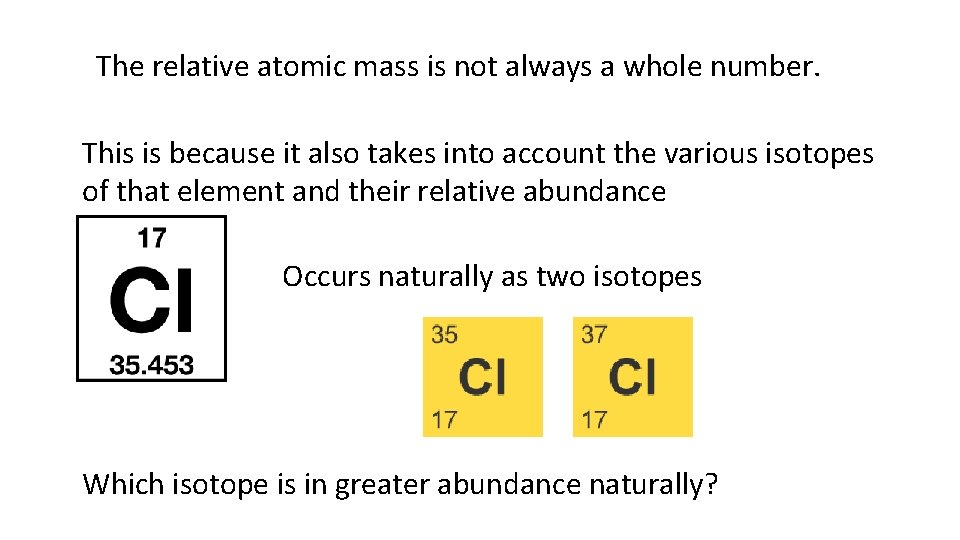
The relative atomic mass is not always a whole number. This is because it also takes into account the various isotopes of that element and their relative abundance Occurs naturally as two isotopes Which isotope is in greater abundance naturally?

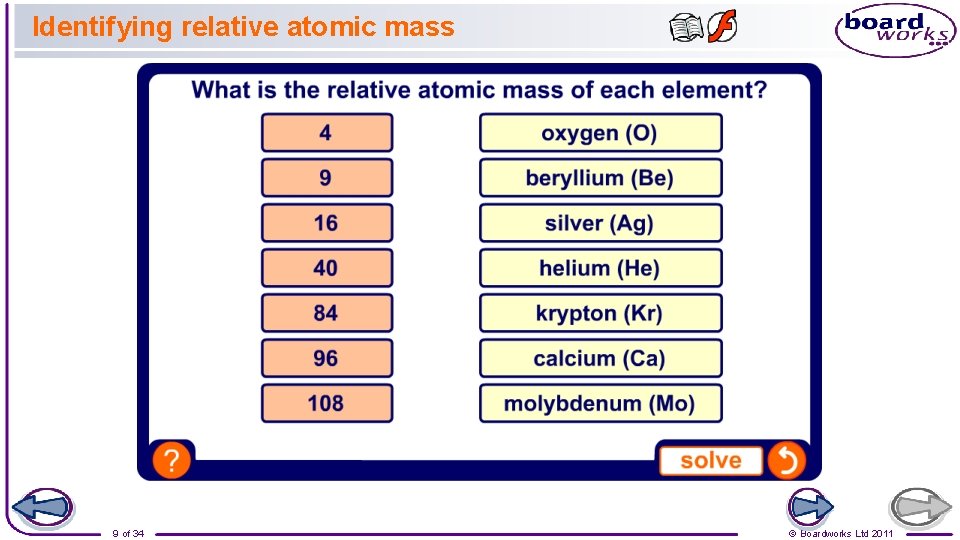
Identifying relative atomic mass 9 of 34 © Boardworks Ltd 2011

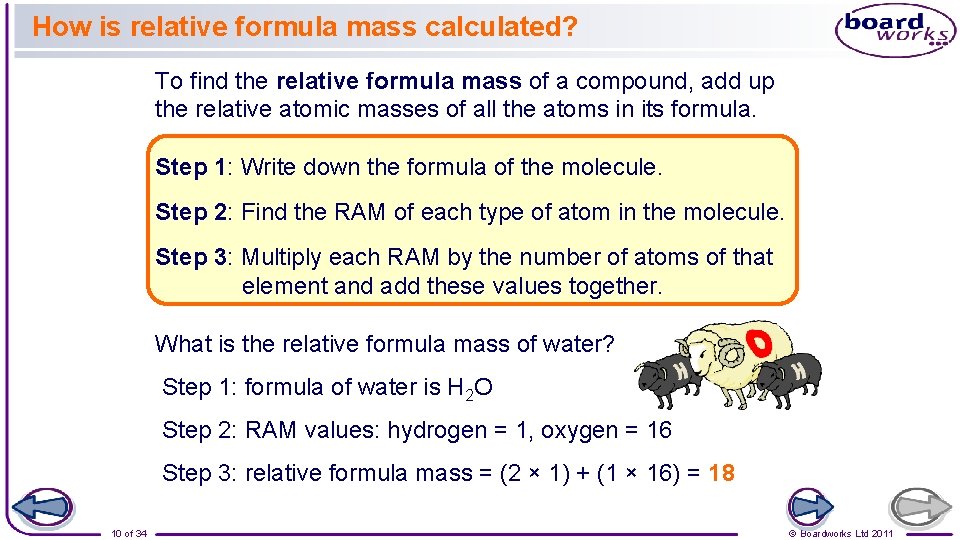
How is relative formula mass calculated? To find the relative formula mass of a compound, add up the relative atomic masses of all the atoms in its formula. Step 1: Write down the formula of the molecule. Step 2: Find the RAM of each type of atom in the molecule. Step 3: Multiply each RAM by the number of atoms of that element and add these values together. What is the relative formula mass of water? Step 1: formula of water is H 2 O Step 2: RAM values: hydrogen = 1, oxygen = 16 Step 3: relative formula mass = (2 × 1) + (1 × 16) = 18 10 of 34 © Boardworks Ltd 2011

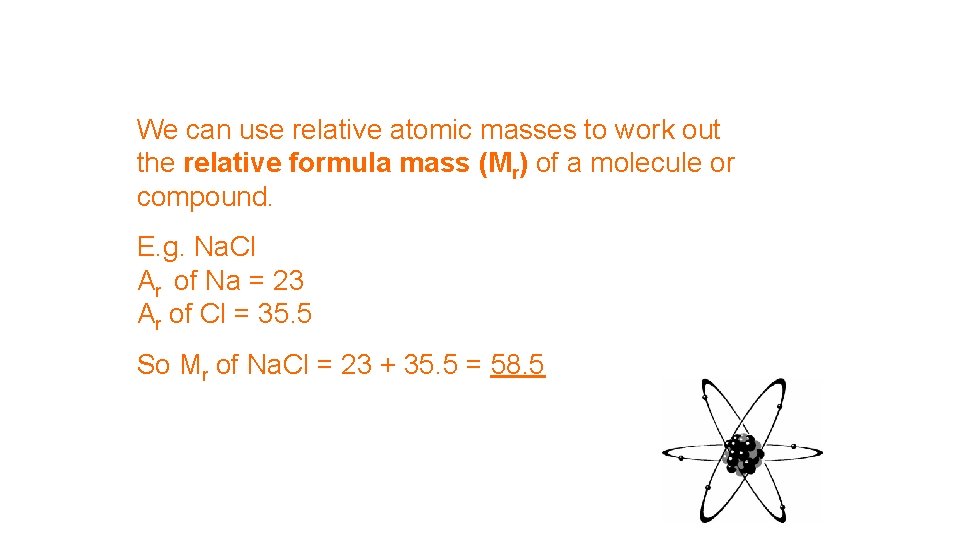
We can use relative atomic masses to work out the relative formula mass (Mr) of a molecule or compound. E. g. Na. Cl Ar of Na = 23 Ar of Cl = 35. 5 So Mr of Na. Cl = 23 + 35. 5 = 58. 5

Calculate the relative formula mass of the following compounds (showing your working!): (a) Carbon monoxide CO (b) Carbon dioxide (c) Sulphur dioxide SO 2 (d) Calcium carbonate Ca. CO 3 (e) Sodium hydroxide Na. OH (f) Sulphuric acid H 2 SO 4 (g) Hydrochloric acid HCl (h) Copper sulphate Cu. SO 4 (i) Magnesium chloride Mg. Cl 2 (j) Sodium carbonate Na 2 CO 3 (k) Lead nitrate Pb(NO 3)2 (l) Calcium hydroxide Ca(OH)2

In a balanced equation, the sum of the relative formula masses of the reactants in the quantities shown is equal to the sum of the relative formula masses of the products in the quantities shown. Check that this is true for: 2 Mg + O 2 2 Mg. O

What is a mole? Since atoms are so small it is impossible to measure out how many individual atoms of a particular element is needed in reactions. Therefore, a unit called the mole was established which allows us to measure out an exact number of atoms.

One mole contains: 6. 02 x 1023 entities (entities can be atoms, molecules etc. ) • One mole of sodium ions contains 6. 02 x 1023 sodium ions • One mole of water contains 6. 02 x 1023 water molecules • One mole of doughnuts contains 6. 02 x 1023 doughnuts

Just How Big is a Mole? • Enough soft drink cans to cover the surface of the earth to a depth of over 200 miles. • If you had Avogadro's number of unpopped popcorn kernels, and spread them across the United States of America, the country would be covered in popcorn to a depth of over 9 miles. • If we were able to count atoms at the rate of 10 million per second, it would take about 2 billion years to count the atoms in one mole. 16

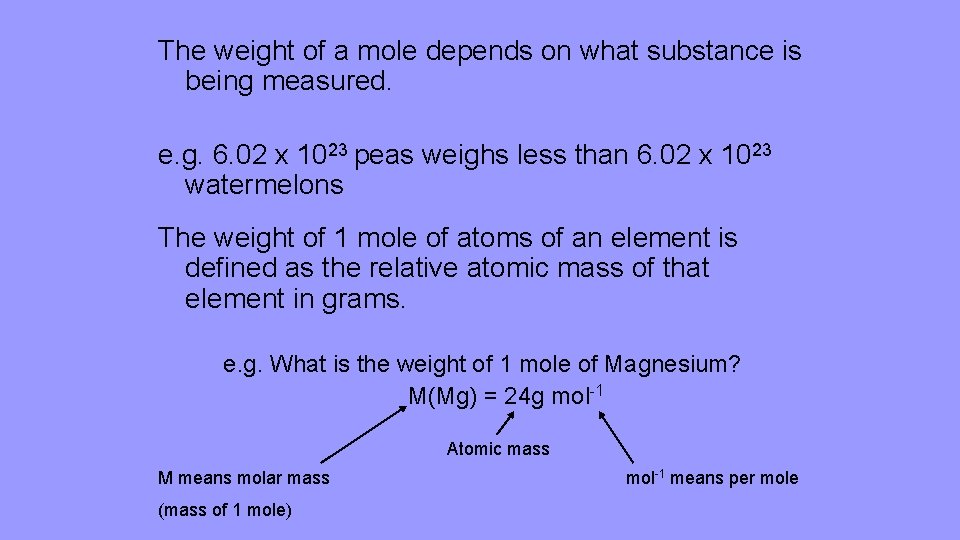
The weight of a mole depends on what substance is being measured. e. g. 6. 02 x 1023 peas weighs less than 6. 02 x 1023 watermelons The weight of 1 mole of atoms of an element is defined as the relative atomic mass of that element in grams. e. g. What is the weight of 1 mole of Magnesium? M(Mg) = 24 g mol-1 Atomic mass M means molar mass (mass of 1 mole) mol-1 means per mole
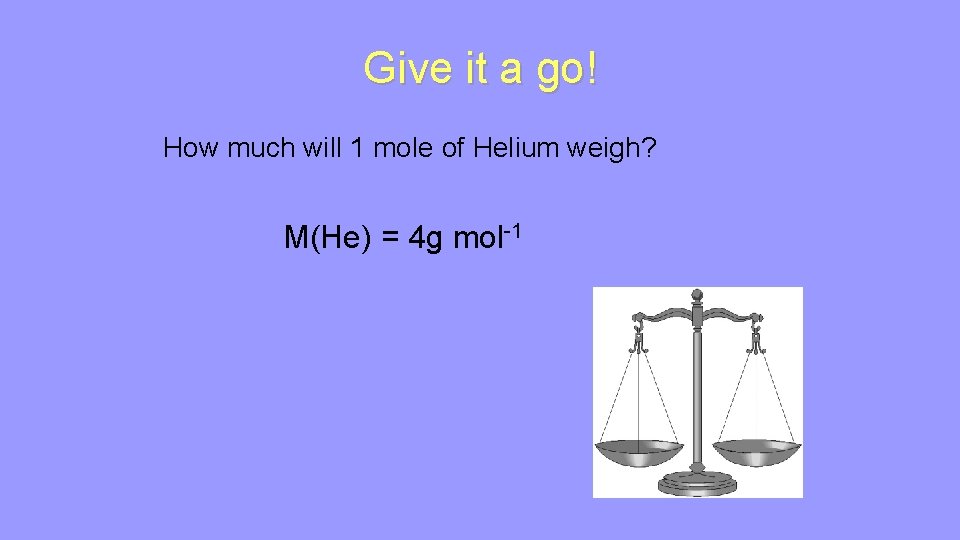
Give it a go! How much will 1 mole of Helium weigh? M(He) = 4 g mol-1

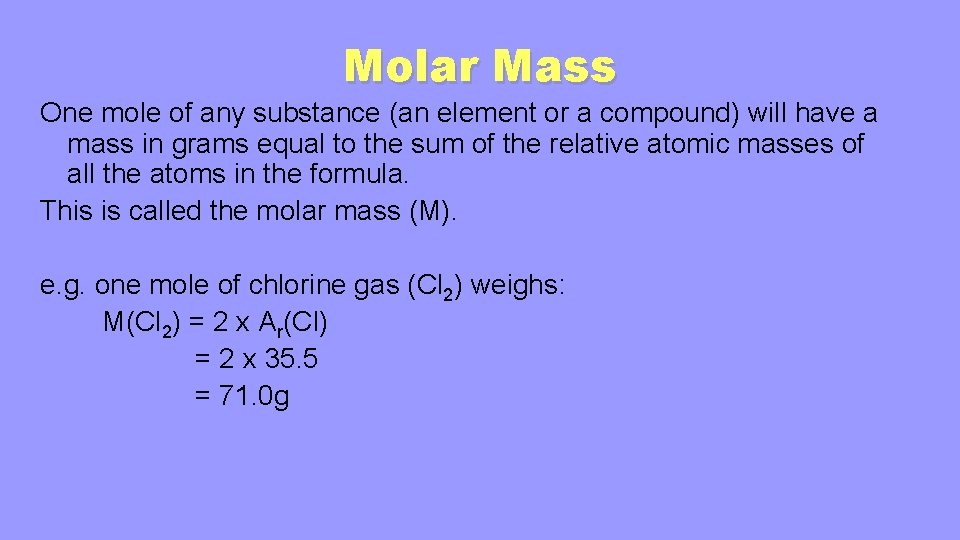
Molar Mass One mole of any substance (an element or a compound) will have a mass in grams equal to the sum of the relative atomic masses of all the atoms in the formula. This is called the molar mass (M). e. g. one mole of chlorine gas (Cl 2) weighs: M(Cl 2) = 2 x Ar(Cl) = 2 x 35. 5 = 71. 0 g

Give it a go! Calculate the Molar mass of hydrogen chloride (HCl). M(HCl) = Ar (H) + Ar (Cl) = 1. 00 + 35. 5 = 36. 5 g

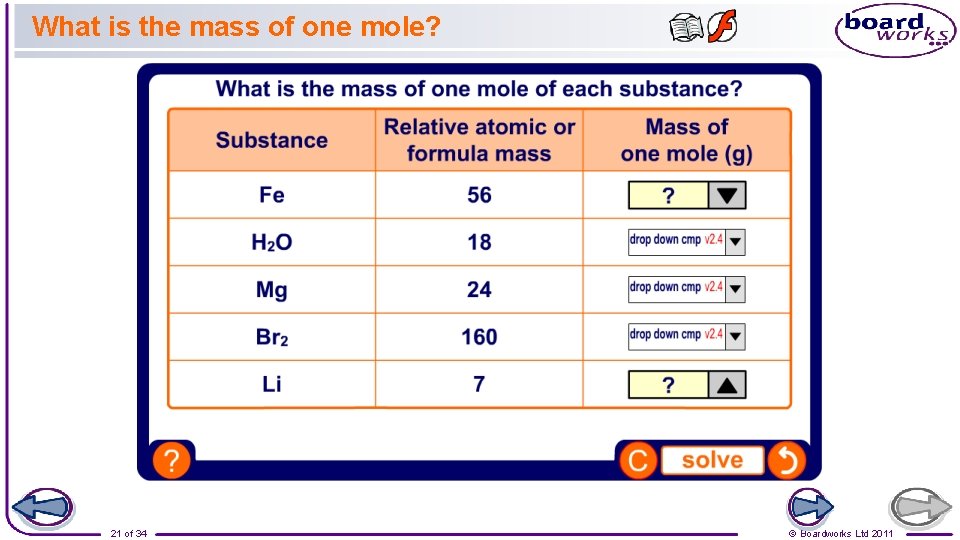
What is the mass of one mole? 21 of 34 © Boardworks Ltd 2011

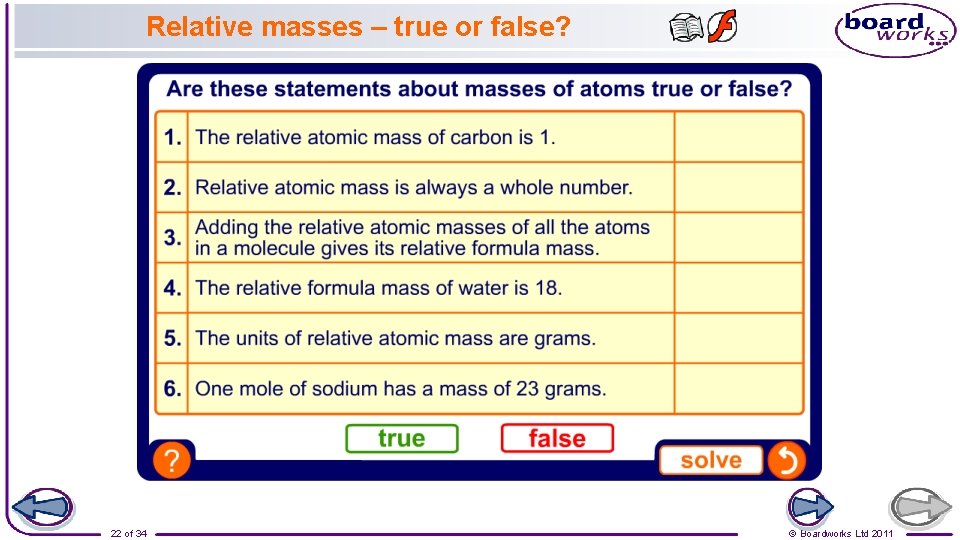
Relative masses – true or false? 22 of 34 © Boardworks Ltd 2011
