Atomic Theory Atomic Structure Periodic Electron Trends Configuration

















- Slides: 28


Atomic Theory Atomic Structure Periodic Electron Trends Configuration Table 100 100 100 200 200 200 300 300 300 400 400 400 500 500 500

? 1 $100 This is where electrons are found in an atom. A. In fixed energy levels B. In the nucleus C. In a probable location in the electron cloud D. In an exact location in the electron cloud

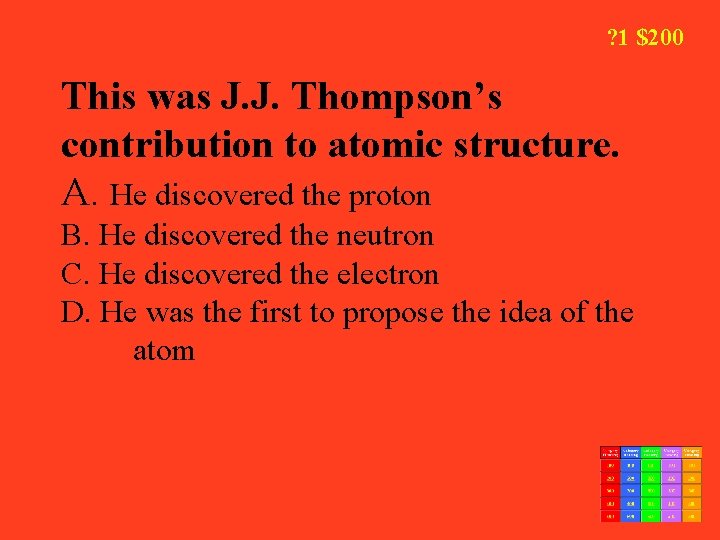
? 1 $200 This was J. J. Thompson’s contribution to atomic structure. A. He discovered the proton B. He discovered the neutron C. He discovered the electron D. He was the first to propose the idea of the atom

? 1 $300 This conclusion made by Dalton was later proven to be wrong. A. Atoms of the same element are identical B. Atoms are indivisible, solid particles C. Elements can physically mix to form mixtures D. Elements can chemically combine to form compounds

? 1 $400 This is not a conclusion that Ernest Rutherford made after performing the gold foil experiment. A. The nucleus has a positive charge B. The nucleus is dense C. The nucleus contains protons, neutrons, and electrons D. The atom is mainly empty space

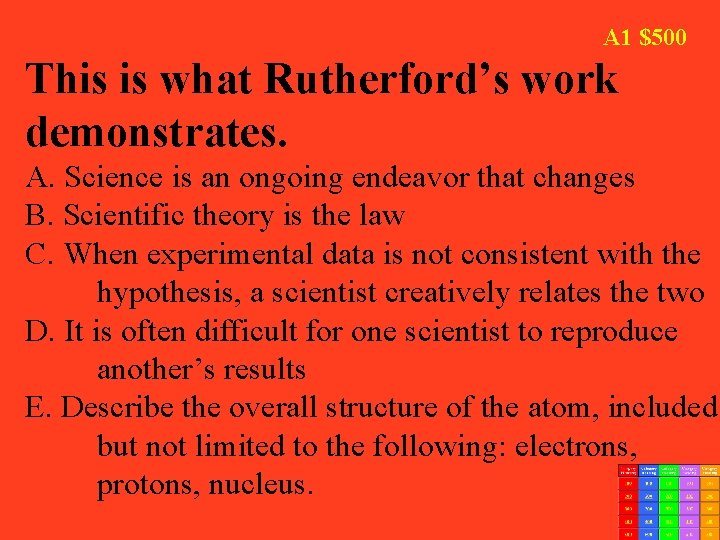
A 1 $500 This is what Rutherford’s work demonstrates. A. Science is an ongoing endeavor that changes B. Scientific theory is the law C. When experimental data is not consistent with the hypothesis, a scientist creatively relates the two D. It is often difficult for one scientist to reproduce another’s results E. Describe the overall structure of the atom, included but not limited to the following: electrons, protons, nucleus.

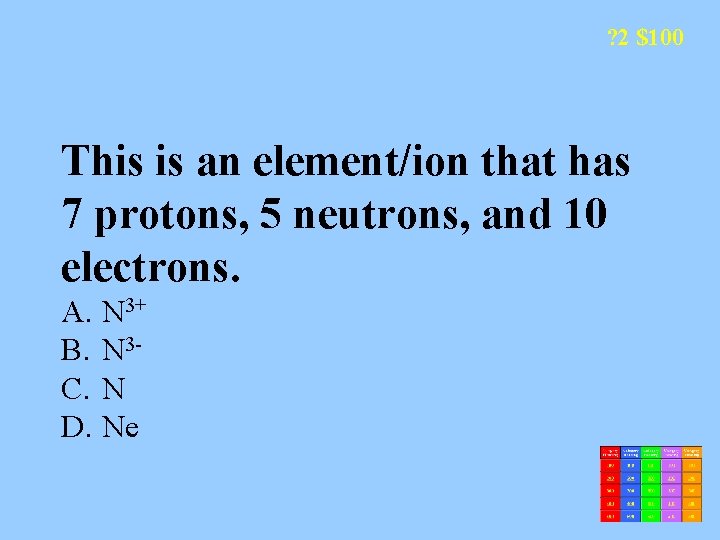
? 2 $100 This is an element/ion that has 7 protons, 5 neutrons, and 10 electrons. A. N 3+ B. N 3 C. N D. Ne

? 2 $200 This is the mass number of an element with 27 protons, 25 electrons, and 27 neutrons. A. 2 B. 52 C. 54 D. 79

? 2 $300 Be 2+ has this number of electrons.

? 2 $400 Two isotopes of arsenic have different numbers of this subatomic particle. A. protons B. neutrons C. electrons D. mass number

? 2 $500 Element X has two isotopes. If 72% of the element has an isotopic mass of 84 atomic mass units, and 28% of the element has an isotopic mass of 87 atomic mass units. This is the average atomic mass of element X. A. 84 amu B. 84. 8 amu C. 85. 5 amu D. 87 amu

? 3 $100 This is the atomic number of the most electronegative element. (Options 1 -10)

This is the order obtained when placing the elements C, N, Si in order from smallest to largest atomic radius. A. C, N, Si B. N, C, Si C. Si, C, N D. Si, N, C E. C, Si, N F. N, Si, C ? 3 $200

? 3 $300 This is the reason why ionization energy decreases down a group. A. Shielding B. Increase in Energy Level C. Metals like to lose electrons

? 3 $400 This property explains why lithium is most likely of the period 2 elements to give up an electron. A. Atomic Radius B. Density C. Electronegativity D. Ionization energy

? 3 $500 This is how metals are different from nonmetals in terms of ionization energies and electronegativies. A. Metals have lower IE and lower electroneg. B. Metals have higher IE and lower electroneg. C. Metals have higher IE and higher electroneg. D. Metals have lower IE and higher electroneg.

? 4 $100 Elements in group 6 A have electron configurations ending in this notation. A. p 2 B. p 3 C. p 4 D. p 5 E. p 6

? 4 $200 This is the electron configuration of bromine. A. 1 s 22 p 63 s 23 p 64 s 23 d 104 p 5 B. 1 s 22 p 63 s 23 p 63 d 104 s 24 p 5 C. 1 s 22 p 63 s 23 p 64 s 23 d 104 p 6 D. 1 s 22 p 43 s 23 p 63 d 84 s 24 p 6

? 4 $300 For the electron configuration 5 s 2, this is what the 5, the s, and the 2 represent. A. principle energy level/period, shape, # e- in sublevel B. principle energy level/group, shape, # e- in sublevel C. principle energy level/period, shape, # e- in element D. principle energy level/group, shape, # e- in element

? 4 $400 This is the number of unpaired electrons Oxygen has.

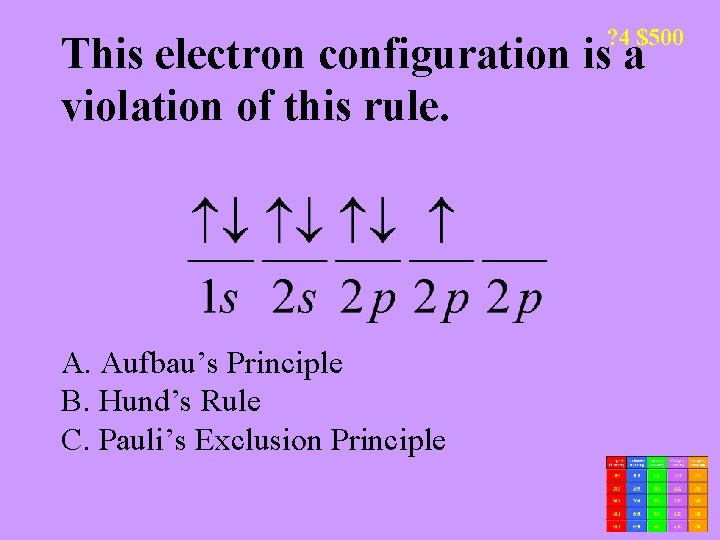
? 4 $500 This electron configuration is a violation of this rule. A. Aufbau’s Principle B. Hund’s Rule C. Pauli’s Exclusion Principle

? 5 $100 The periodic law is applicable when elements are arranged by this. A. Increasing atomic mass B. Increasing mass number C. Increasing atomic number D. Decreasing atomic mass

? 5 $200 These elements are generally nonreactive. A. Alkali metals B. Alkaline earth metals C. Transition metals D. Inner transition metals E. Metalloids F. Halogens G. Noble gases

? 5 $300 This is not a property of a metal at room temperature. A. Conducts electricity B. Lustrous C. Malleable D. Gas

? 5 $400 Halogens have this number of valence electrons.

The element in period 2 that has the largest atomic radius is a member of this family of elements. ? 5 $500 A. Alkali metals B. Alkaline earth metals C. Transition metals D. Inner transition metals E. Metalloids F. Halogens G. Noble gases

FINAL JEOPARDY 1. Identify the element based upon the following quantum numbers: (4, 2, 1, ½) 2. Write the four quantum numbers of the following element: Xe (#54)