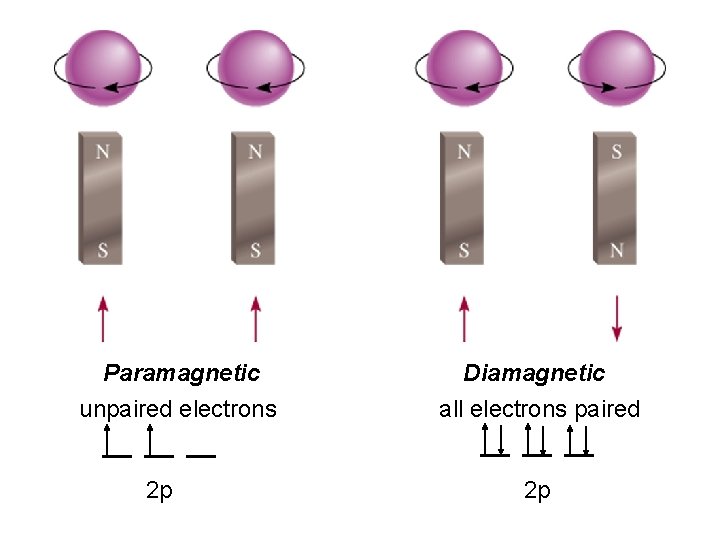
Paramagnetic unpaired electrons 2 p Diamagnetic all electrons

![Roentgenium 111 Rg: [Rn] 7 s 2 5 f 14 6 d 9 111 Roentgenium 111 Rg: [Rn] 7 s 2 5 f 14 6 d 9 111](https://slidetodoc.com/presentation_image/053678109877bd7f21b03df355c74c97/image-12.jpg)


![(1) Write the ground state electron configuration for the selenium atom. A. [Ar] 4 (1) Write the ground state electron configuration for the selenium atom. A. [Ar] 4](https://slidetodoc.com/presentation_image/053678109877bd7f21b03df355c74c97/image-18.jpg)


- Slides: 22


Paramagnetic unpaired electrons 2 p Diamagnetic all electrons paired 2 p



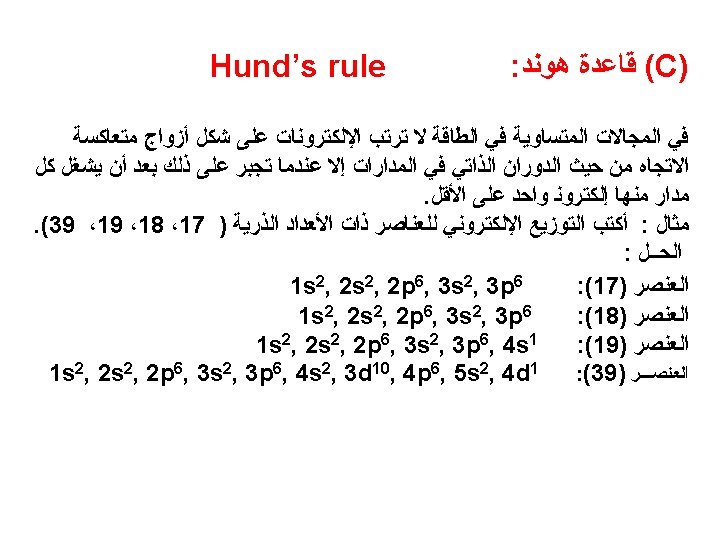
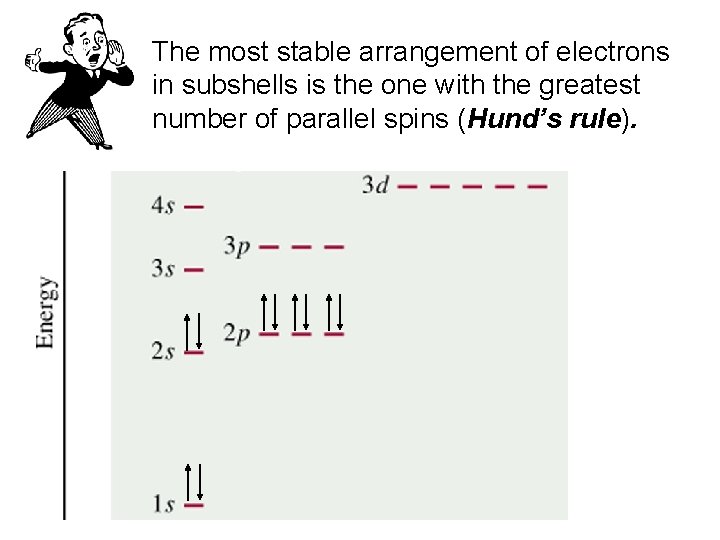
The most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins (Hund’s rule).


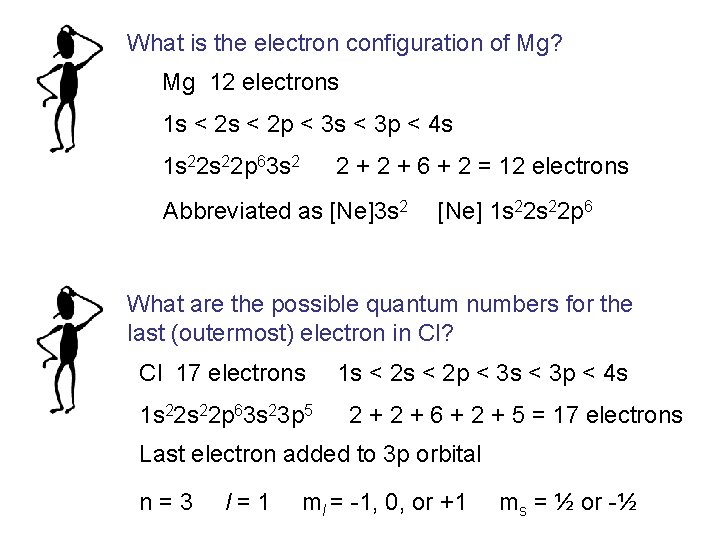
What is the electron configuration of Mg? Mg 12 electrons 1 s < 2 p < 3 s < 3 p < 4 s 1 s 22 p 63 s 2 2 + 6 + 2 = 12 electrons Abbreviated as [Ne]3 s 2 [Ne] 1 s 22 p 6 What are the possible quantum numbers for the last (outermost) electron in Cl? Cl 17 electrons 1 s 22 p 63 s 23 p 5 1 s < 2 p < 3 s < 3 p < 4 s 2 + 6 + 2 + 5 = 17 electrons Last electron added to 3 p orbital n = 3 l = 1 ml = -1, 0, or +1 ms = ½ or -½

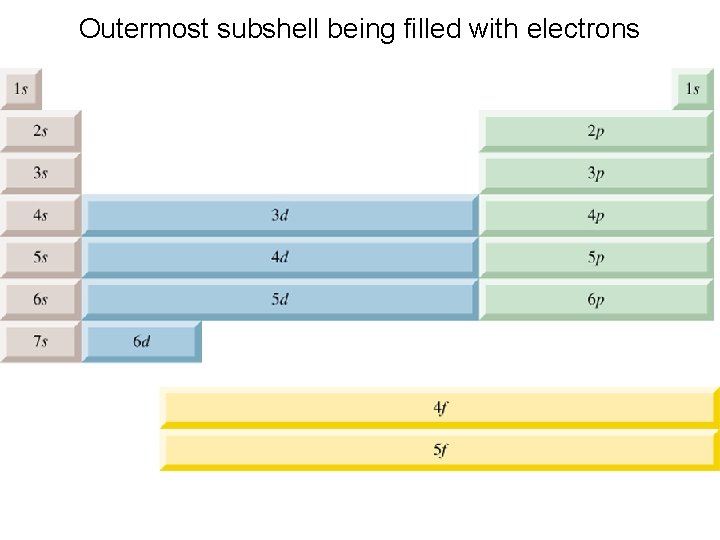
Outermost subshell being filled with electrons


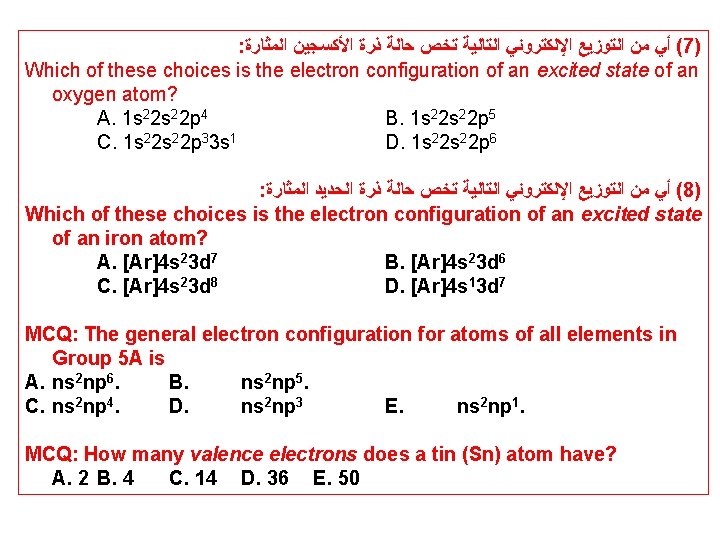
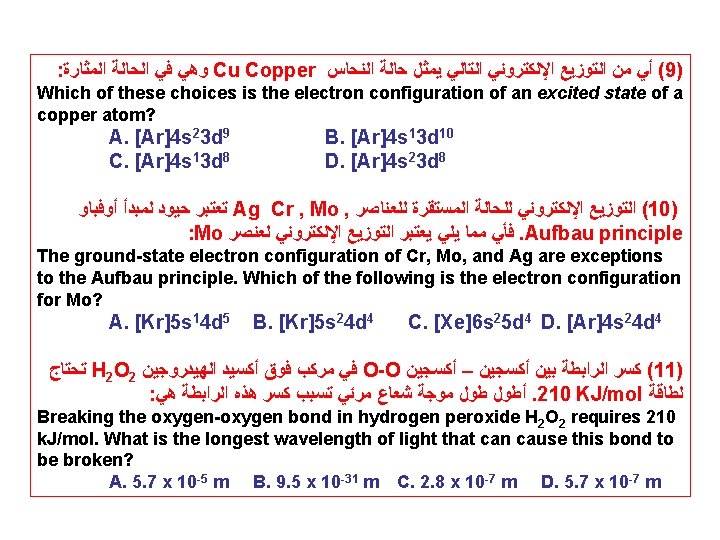
: ﺍﻟﻤﺜﺎﺭﺓ ﺍﻷﻜﺴﺠﻴﻦ ﺫﺭﺓ ﺣﺎﻟﺔ ﺗﺨﺺ ﺍﻟﺘﺎﻟﻴﺔ ﺍﻹﻟﻜﺘﺮﻭﻧﻲ ﺍﻟﺘﻮﺯﻳﻊ ﻣﻦ ﺃﻲ (7) Which of these choices is the electron configuration of an excited state of an oxygen atom? A. 1 s 22 p 4 B. 1 s 22 p 5 C. 1 s 22 p 33 s 1 D. 1 s 22 p 6 : ﺍﻟﻤﺜﺎﺭﺓ ﺍﻟﺤﺪﻳﺪ ﺫﺭﺓ ﺣﺎﻟﺔ ﺗﺨﺺ ﺍﻟﺘﺎﻟﻴﺔ ﺍﻹﻟﻜﺘﺮﻭﻧﻲ ﺍﻟﺘﻮﺯﻳﻊ ﻣﻦ ﺃﻲ (8) Which of these choices is the electron configuration of an excited state of an iron atom? A. [Ar]4 s 23 d 7 B. [Ar]4 s 23 d 6 C. [Ar]4 s 23 d 8 D. [Ar]4 s 13 d 7 MCQ: The general electron configuration for atoms of all elements in Group 5 A is A. ns 2 np 6. B. ns 2 np 5. C. ns 2 np 4. D. ns 2 np 3 E. ns 2 np 1. MCQ: How many valence electrons does a tin (Sn) atom have? A. 2 B. 4 C. 14 D. 36 E. 50

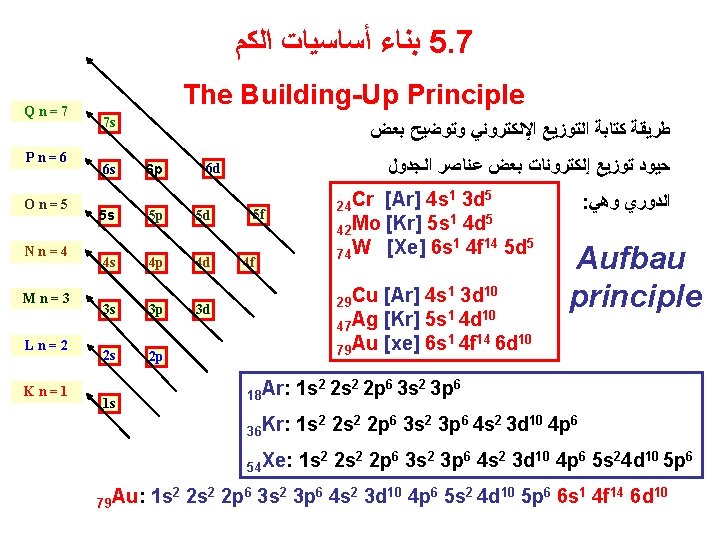
ﺍﻟﻜﻢ ﺃﺴﺎﺳﻴﺎﺕ ﺑﻨﺎﺀ 5. 7 Qn=7 Pn=6 On=5 Nn=4 Mn=3 Ln=2 Kn=1 The Building-Up Principle 7 s 6 s 5 s ﺑﻌﺾ ﻭﺗﻮﺿﻴﺢ ﺍﻹﻟﻜﺘﺮﻭﻧﻲ ﺍﻟﺘﻮﺯﻳﻊ ﻛﺘﺎﺑﺔ ﻃﺮﻳﻘﺔ 6 p 5 p 5 d 4 s 4 p 4 d 3 s 3 p 3 d 2 s 2 p 1 s ﺍﻟﺠﺪﻭﻝ ﻋﻨﺎﺻﺮ ﺑﻌﺾ ﺇﻟﻜﺘﺮﻭﻧﺎﺕ ﺗﻮﺯﻳﻊ ﺣﻴﻮﺩ 6 d 24 Cr [Ar] 4 s 5 f 42 Mo [Kr] 5 s 1 4 d 5 74 W [Xe] 6 s 4 f 14 5 d 5 1 10 29 Cu [Ar] 4 s 3 d 1 10 47 Ag [Kr] 5 s 4 d 1 14 10 79 Au [xe] 6 s 4 f 6 d : ﻭﻫﻲ ﺍﻟﺪﻭﺭﻱ Aufbau principle 18 Ar: 1 s 2 2 p 6 3 s 2 3 p 6 36 Kr: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 54 Xe: 1 s 79 Au: 1 s 1 3 d 5 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 24 d 10 5 p 6 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 1 4 f 14 6 d 10
![Roentgenium 111 Rg Rn 7 s 2 5 f 14 6 d 9 111 Roentgenium 111 Rg: [Rn] 7 s 2 5 f 14 6 d 9 111](https://slidetodoc.com/presentation_image/053678109877bd7f21b03df355c74c97/image-12.jpg)
Roentgenium 111 Rg: [Rn] 7 s 2 5 f 14 6 d 9 111 Rg: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 Qn=7 7 s Pn=6 6 s 6 p On=5 5 s 5 p 5 d Nn=4 4 s 4 p 4 d Mn=3 3 s 3 p 3 d Ln=2 2 s 2 p Kn=1 1 s 4 d 10 5 p 6 6 s 2 4 f 14 5 d 10 6 p 6 7 s 2 5 f 14 6 d 9 1 H: 1 s 1 Page 300 2 1 s 1 Li: 1 s 3 11 Na: 1 s 19 K: 1 s 2 2 p 6 2 s 1 2 2 s 2 2 p 6 3 s 2 3 p 6 Ref: Chang 4 s 1 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 1 Rb: 1 s 37 55 Cs: 1 s 87 Fr: 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 d 6 s 1 2 2 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 14 5 d 10 6 p 6 87 Fr; [Rn] 7 s 1 Fr : Francium Rn : Radon 7 s 1 5 f 4 f

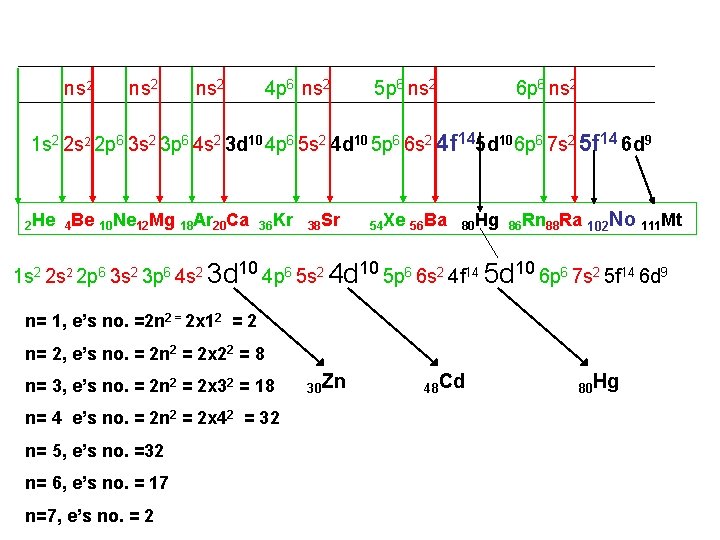
ns 2 4 p 6 ns 2 5 p 6 ns 2 6 p 6 ns 2 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 145 d 106 p 6 7 s 2 5 f 14 6 d 9 2 He 4 Be 10 Ne 12 Mg 18 Ar 20 Ca 36 Kr 38 Sr 54 Xe 56 Ba 80 Hg 86 Rn 88 Ra 102 No 111 Mt 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 14 5 d 10 6 p 6 7 s 2 5 f 14 6 d 9 n= 1, e’s no. =2 n 2 = 2 x 12 = 2 n= 2, e’s no. = 2 n 2 = 2 x 22 = 8 n= 3, e’s no. = 2 n 2 = 2 x 32 = 18 n= 4 e’s no. = 2 n 2 = 2 x 42 = 32 n= 5, e’s no. =32 n= 6, e’s no. = 17 n=7, e’s no. = 2 30 Zn 48 Cd 80 Hg

1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 145 d 106 p 6 7 s 2 5 f 14 6 d 9

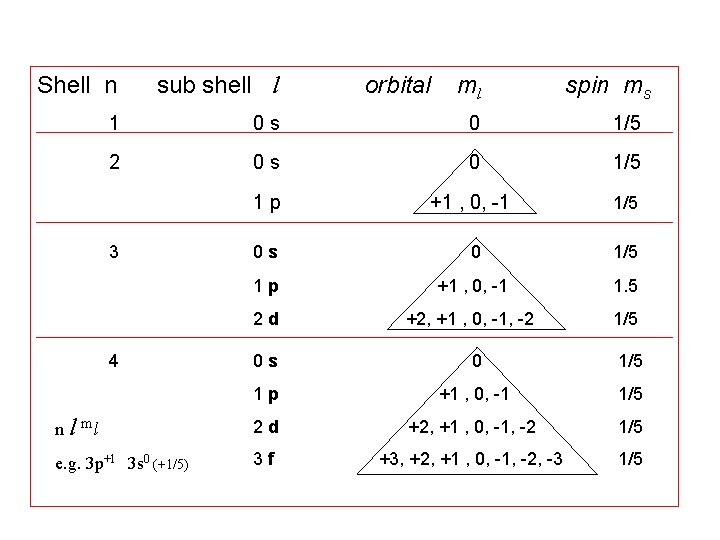
Shell n sub shell l orbital ml spin ms 1 0 s 0 1/5 2 0 s 0 1/5 1 p +1 , 0, -1 1/5 0 s 1/5 1 p +1 , 0, -1 2 d +2, +1 , 0, -1, -2 1/5 0 s 0 1/5 1 p +1 , 0, -1 1/5 n l ml 2 d e. g. 3 p+1 3 s 0 (+1/5) 3 f 3 4 0 +2, +1 , 0, -1, -2 +3, +2, +1 , 0, -1, -2, -3 1. 5 1/5


: ﺍﻟﻤﺜﺎﺭﺓ ﺍﻟﺤﺎﻟﺔ ﻓﻲ ﻭﻫﻲ Cu Copper ﺍﻟﻨﺤﺎﺱ ﺣﺎﻟﺔ ﻳﻤﺜﻞ ﺍﻟﺘﺎﻟﻲ ﺍﻹﻟﻜﺘﺮﻭﻧﻲ ﺍﻟﺘﻮﺯﻳﻊ ﻣﻦ ﺃﻲ (9) Which of these choices is the electron configuration of an excited state of a copper atom? A. [Ar]4 s 23 d 9 B. [Ar]4 s 13 d 10 C. [Ar]4 s 13 d 8 D. [Ar]4 s 23 d 8 ﺃﻮﻓﺒﺎﻭ ﻟﻤﺒﺪﺃ ﺣﻴﻮﺩ ﺗﻌﺘﺒﺮ Ag Cr , Mo , ﻟﻠﻌﻨﺎﺻﺮ ﺍﻟﻤﺴﺘﻘﺮﺓ ﻟﻠﺤﺎﻟﺔ ﺍﻹﻟﻜﺘﺮﻭﻧﻲ ﺍﻟﺘﻮﺯﻳﻊ (10) : Mo ﻟﻌﻨﺼﺮ ﺍﻹﻟﻜﺘﺮﻭﻧﻲ ﺍﻟﺘﻮﺯﻳﻊ ﻳﻌﺘﺒﺮ ﻳﻠﻲ ﻣﻤﺎ ﻓﺄﻲ . Aufbau principle The ground-state electron configuration of Cr, Mo, and Ag are exceptions to the Aufbau principle. Which of the following is the electron configuration for Mo? A. [Kr]5 s 14 d 5 B. [Kr]5 s 24 d 4 C. [Xe]6 s 25 d 4 D. [Ar]4 s 24 d 4 ﺗﺤﺘﺎﺝ H 2 O 2 ﺍﻟﻬﻴﺪﺭﻭﺟﻴﻦ ﺃﻜﺴﻴﺪ ﻓﻮﻕ ﻣﺮﻛﺐ ﻓﻲ O-O ﺃﻜﺴﺠﻴﻦ – ﺃﻜﺴﺠﻴﻦ ﺑﻴﻦ ﺍﻟﺮﺍﺑﻄﺔ ﻛﺴﺮ (11) : ﻫﻲ ﺍﻟﺮﺍﺑﻄﺔ ﻫﺬﻩ ﻛﺴﺮ ﺗﺴﺒﺐ ﻣﺮﺋﻲ ﺷﻌﺎﻉ ﻣﻮﺟﺔ ﻃﻮﻝ ﺃﻄﻮﻝ . 210 KJ/mol ﻟﻄﺎﻗﺔ Breaking the oxygen-oxygen bond in hydrogen peroxide H 2 O 2 requires 210 k. J/mol. What is the longest wavelength of light that can cause this bond to be broken? A. 5. 7 x 10 -5 m B. 9. 5 x 10 -31 m C. 2. 8 x 10 -7 m D. 5. 7 x 10 -7 m
![1 Write the ground state electron configuration for the selenium atom A Ar 4 (1) Write the ground state electron configuration for the selenium atom. A. [Ar] 4](https://slidetodoc.com/presentation_image/053678109877bd7f21b03df355c74c97/image-18.jpg)
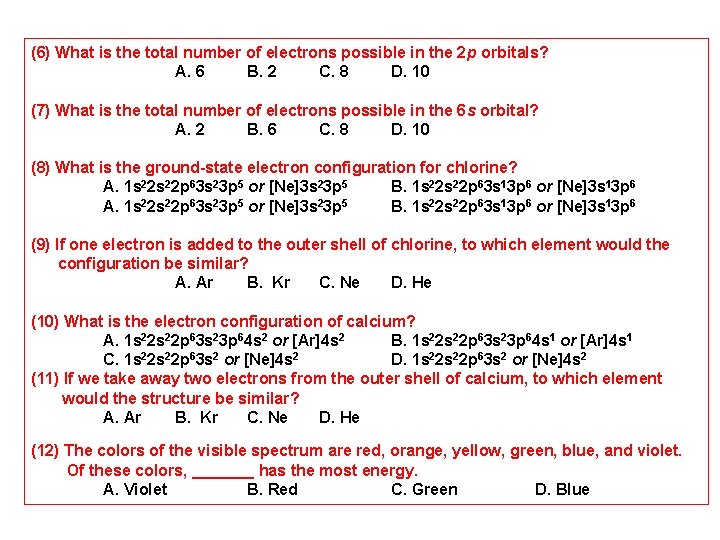
(1) Write the ground state electron configuration for the selenium atom. A. [Ar] 4 s 23 d 104 p 4 B. [Ne] 4 s 23 d 104 p 4 C. [Ar] 4 s 24 d 104 p 4 D. [Ar] 4 s 23 d 105 p 4 (2) Write the ground state electron configuration for the phosphorus atom. A. [Ne] 3 s 23 p 3 B. [Ne] 4 s 24 p 3 C. [Ar] 3 s 23 p 3 D. [He] 3 s 23 p 3 (3) Calculate the energy of a photon of light with a wavelength of 360 nm. A. 5. 5 10 -19 J B. 5. 5 1019 J C. 2. 2 10 -10 J D. 5. 5 1010 J (4) What is the difference in the electron configuration between carbon-14 and carbon 12? A. There is no difference. B. There is difference by 2. C. There is difference by 1. D. There is difference by 3. (5) With regard to electron behavior, what happens when light is absorbed or emitted by an atom? A. The electrons move between orbitals. B. The electrons did not move between orbitals. C. The electrons move between orbitals and in side nucleus. D. All Answers are correct.

(6) What is the total number of electrons possible in the 2 p orbitals? A. 6 B. 2 C. 8 D. 10 (7) What is the total number of electrons possible in the 6 s orbital? A. 2 B. 6 C. 8 D. 10 (8) What is the ground-state electron configuration for chlorine? A. 1 s 22 s 22 p 63 s 23 p 5 or [Ne]3 s 23 p 5 B. 1 s 22 s 22 p 63 s 13 p 6 or [Ne]3 s 13 p 6 (9) If one electron is added to the outer shell of chlorine, to which element would the configuration be similar? A. Ar B. Kr C. Ne D. He (10) What is the electron configuration of calcium? A. 1 s 22 p 63 s 23 p 64 s 2 or [Ar]4 s 2 B. 1 s 22 p 63 s 23 p 64 s 1 or [Ar]4 s 1 C. 1 s 22 p 63 s 2 or [Ne]4 s 2 D. 1 s 22 p 63 s 2 or [Ne]4 s 2 (11) If we take away two electrons from the outer shell of calcium, to which element would the structure be similar? A. Ar B. Kr C. Ne D. He (12) The colors of the visible spectrum are red, orange, yellow, green, blue, and violet. Of these colors, _______ has the most energy. A. Violet B. Red C. Green D. Blue

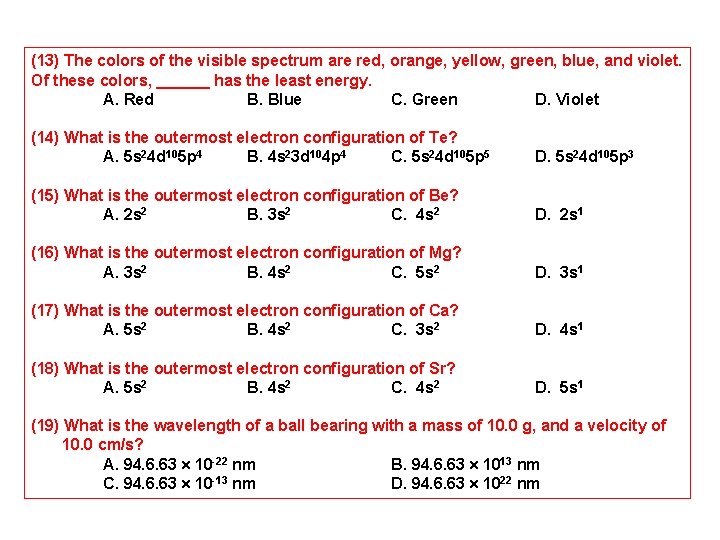
(13) The colors of the visible spectrum are red, orange, yellow, green, blue, and violet. Of these colors, ______ has the least energy. A. Red B. Blue C. Green D. Violet (14) What is the outermost electron configuration of Te? A. 5 s 24 d 105 p 4 B. 4 s 23 d 104 p 4 C. 5 s 24 d 105 p 5 D. 5 s 24 d 105 p 3 (15) What is the outermost electron configuration of Be? A. 2 s 2 B. 3 s 2 C. 4 s 2 D. 2 s 1 (16) What is the outermost electron configuration of Mg? A. 3 s 2 B. 4 s 2 C. 5 s 2 D. 3 s 1 (17) What is the outermost electron configuration of Ca? A. 5 s 2 B. 4 s 2 C. 3 s 2 D. 4 s 1 (18) What is the outermost electron configuration of Sr? A. 5 s 2 B. 4 s 2 C. 4 s 2 D. 5 s 1 (19) What is the wavelength of a ball bearing with a mass of 10. 0 g, and a velocity of 10. 0 cm/s? A. 94. 6. 63 10 -22 nm B. 94. 6. 63 1013 nm C. 94. 6. 63 10 -13 nm D. 94. 6. 63 1022 nm

(20) The bonds of oxygen molecules are broken by sunlight. The minimum energy required to break the oxygen-oxygen bond is 495 k. J/mol. The wavelength of sunlight that can cause this bond breakage is: A. 242 nm B. 424 nm C. 121 nm D. 240 nm : ﻫﻮ d ﺍﻟﻤﺴﺘﻮﻯ ﻓﻲ ﻣﻠﻴﺀ ﺍﻟﻨﺼﻒ ﺍﻷﻴﻮﻥ (21) 30 Zn 2+ ( )ﺩ 25 Mn 2+ ( )ﺝ 27 Co 2+ ( )ﺏ 22 Ti 2+ ( )ﺃ (21) The Bohr model of the hydrogen atom found its greatest support in experimental work on the photoelectric effect. True False (22) An electron in a 3 p orbital could have a value of 2 for its angular momentum quantum number (l). True False (23) A neon atom in its ground state will be diamagnetic. True False (24) Each shell (principal energy level) of quantum number n contains l subshells. True False (25) For all atoms of the same element, the 2 s orbital is larger than the 1 s orbital. True False (26) According to de Broglie's equation, the wavelength associated with the motion of a particle increases as the particle mass decreases. True False (27) The frequency of the emitted light from a cesium atom is an intensive property. True False
