Atomic Structure Modern Atomic Theory Modern Atomic Theory





- Slides: 19

Atomic Structure Modern Atomic Theory

Modern Atomic Theory The atom consists of positive protons, negative electrons, and neutral neutrons. Protons and neutrons are located in the nucleus of the atom, which is small and massive. Electrons are located outside of the nucleus, which creates the volume of the atom.

The Modern View of Atomic Structure

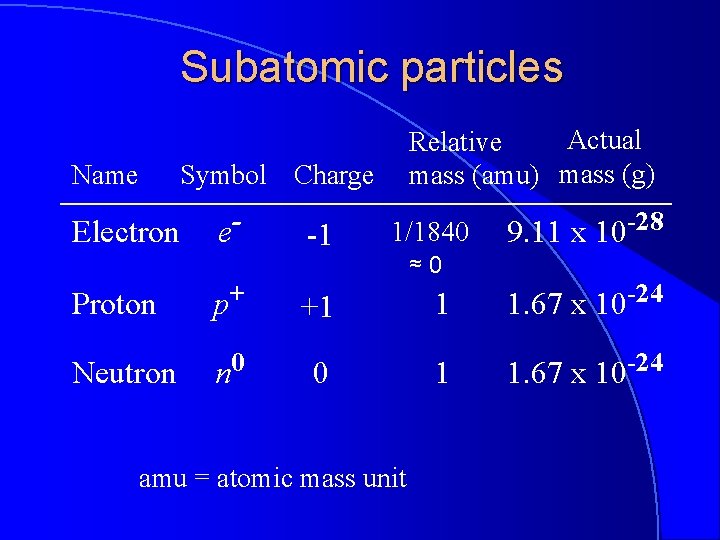
Subatomic particles Name Actual Relative mass (amu) mass (g) Symbol Charge Electron e- Proton p+ Neutron n 0 -1 1/1840 ≈0 9. 11 x 10 -28 +1 1 1. 67 x 10 -24 0 1 1. 67 x 10 -24 amu = atomic mass unit

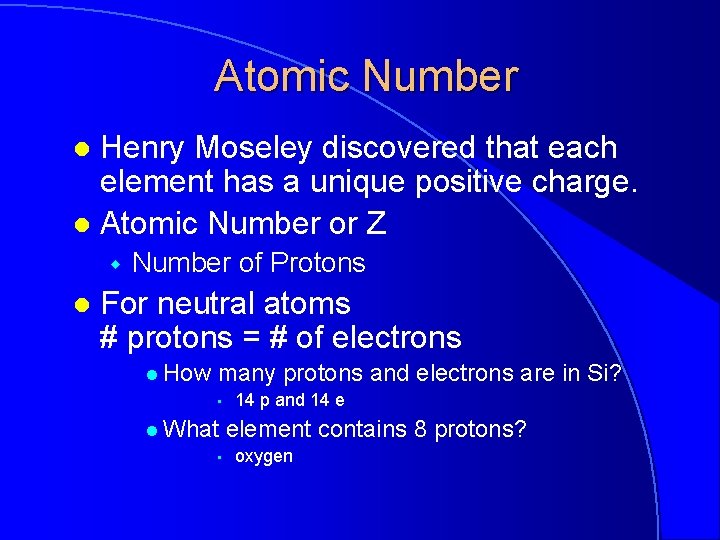
Atomic Number Henry Moseley discovered that each element has a unique positive charge. l Atomic Number or Z l w l Number of Protons For neutral atoms # protons = # of electrons l How many protons and electrons are in Si? • l What • 14 p and 14 e element contains 8 protons? oxygen

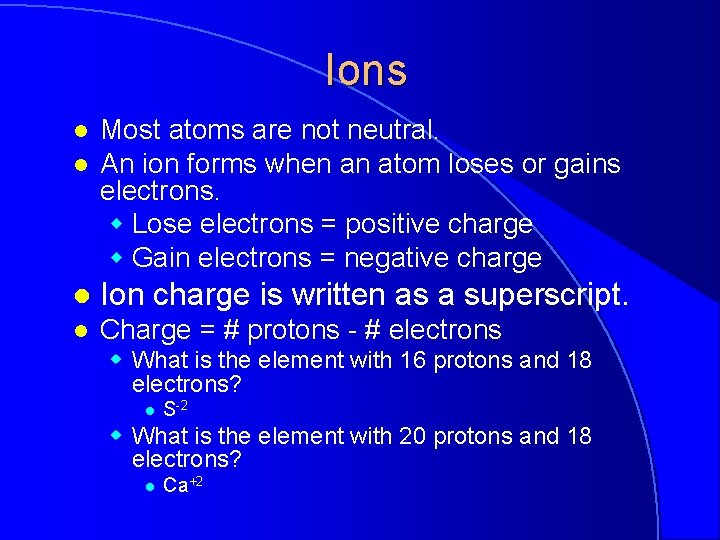
Ions l l Most atoms are not neutral. An ion forms when an atom loses or gains electrons. w Lose electrons = positive charge w Gain electrons = negative charge l Ion charge is written as a superscript. l Charge = # protons - # electrons w What is the element with 16 protons and 18 electrons? l S-2 w What is the element with 20 protons and 18 electrons? l Ca+2

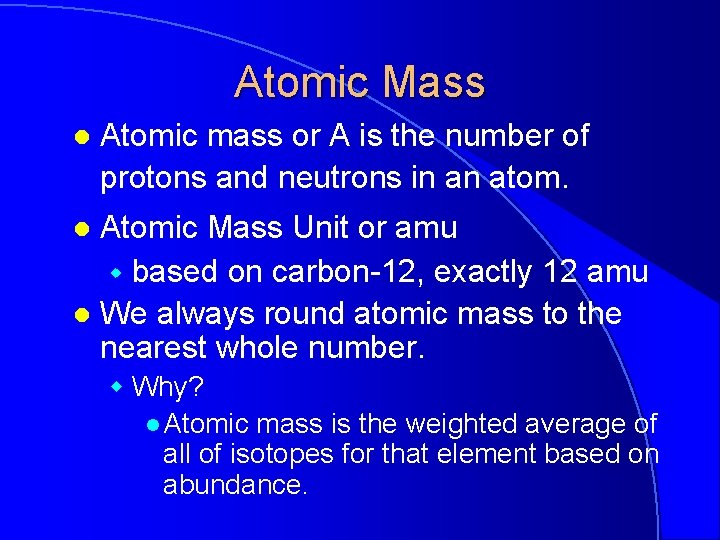
Atomic Mass l Atomic mass or A is the number of protons and neutrons in an atom. Atomic Mass Unit or amu w based on carbon-12, exactly 12 amu l We always round atomic mass to the nearest whole number. l w Why? l Atomic mass is the weighted average of all of isotopes for that element based on abundance.

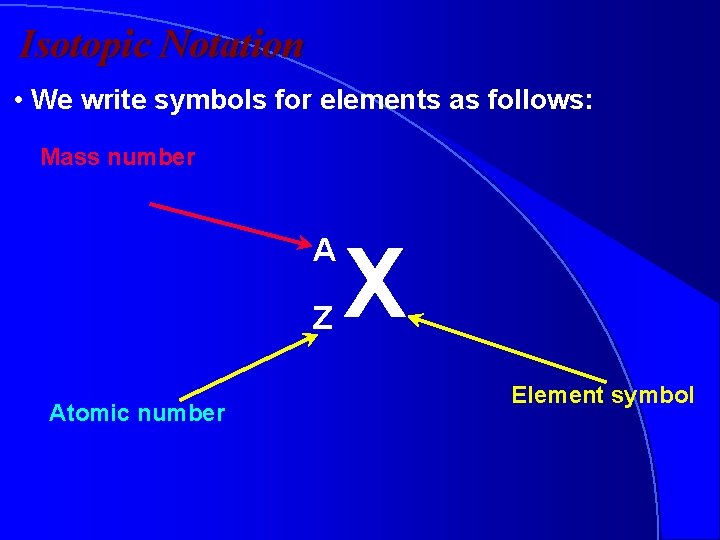
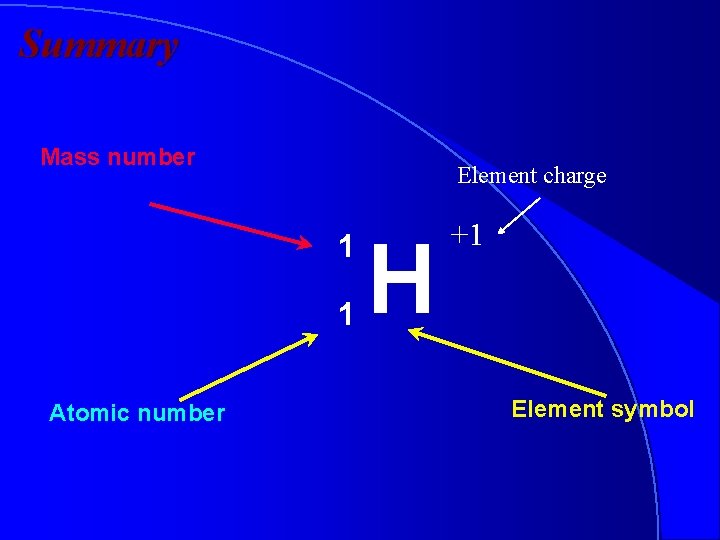
Isotopic Notation • We write symbols for elements as follows: Mass number A Z Atomic number X Element symbol

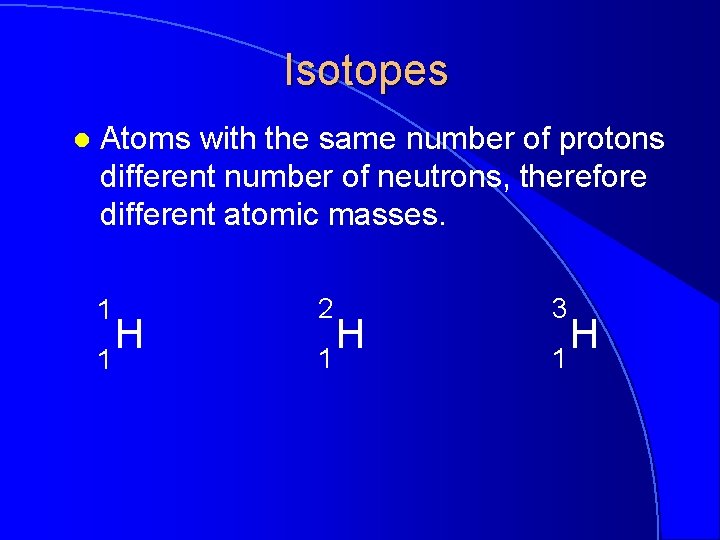
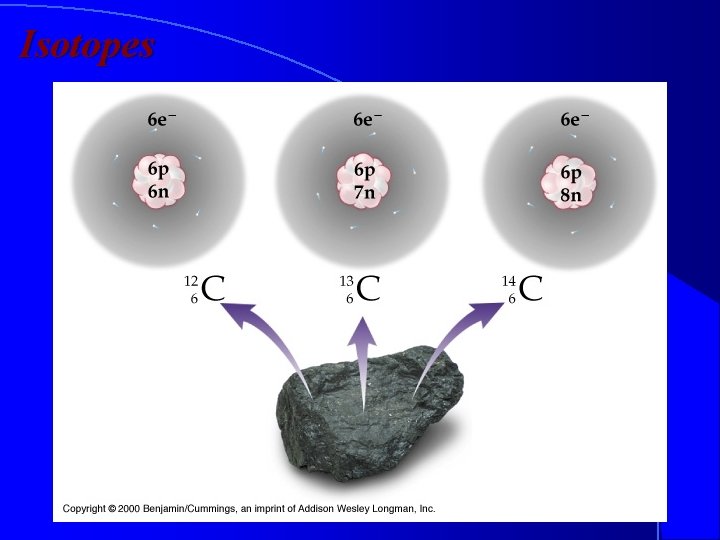
Isotopes l Atoms with the same number of protons different number of neutrons, therefore different atomic masses. 1 1 H 2 1 H 3 1 H

Isotopes

Naming Isotopes l We can also put the mass number after the name of the element. l carbon- 12 l carbon -14 l uranium-235

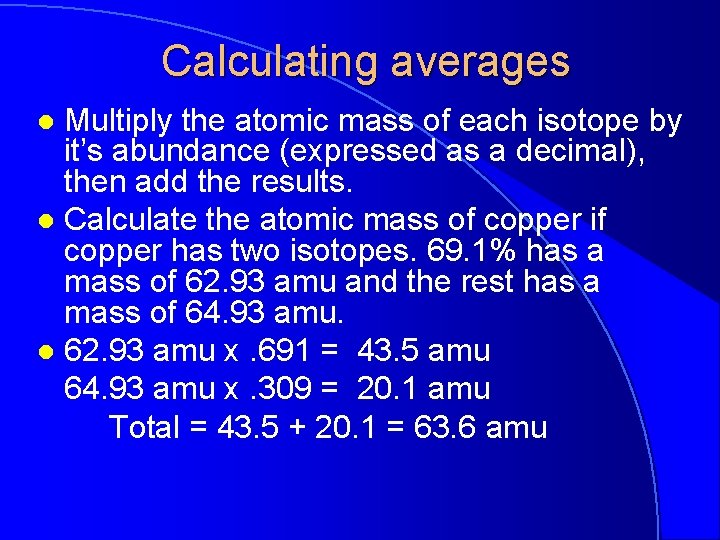
Calculating averages Multiply the atomic mass of each isotope by it’s abundance (expressed as a decimal), then add the results. l Calculate the atomic mass of copper if copper has two isotopes. 69. 1% has a mass of 62. 93 amu and the rest has a mass of 64. 93 amu. l 62. 93 amu x. 691 = 43. 5 amu 64. 93 amu x. 309 = 20. 1 amu Total = 43. 5 + 20. 1 = 63. 6 amu l

Atomic Mass What is the atomic mass of Na? 23 amu l What is the atomic number of Na? 11 amu l Write this in isotopic notation: 23 l Na 11 l To find the neutrons subtract the two numbers. 23 -11 = 12 amu

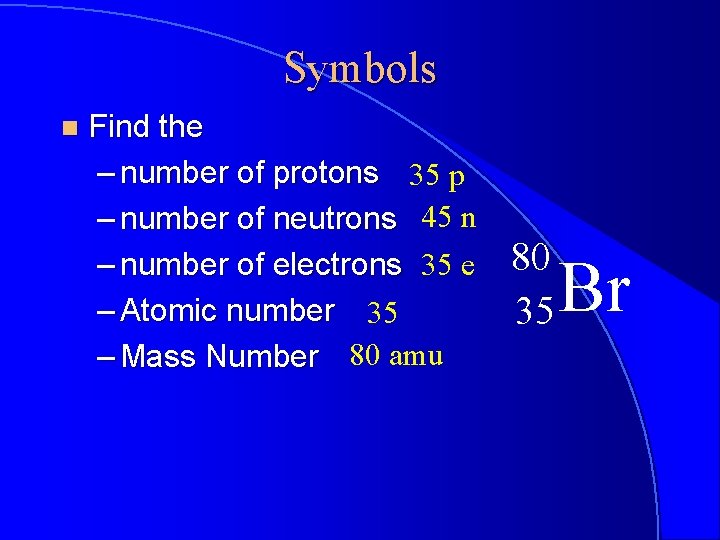
Symbols n Find the – number of protons 35 p – number of neutrons 45 n – number of electrons 35 e – Atomic number 35 – Mass Number 80 amu 80 35 Br

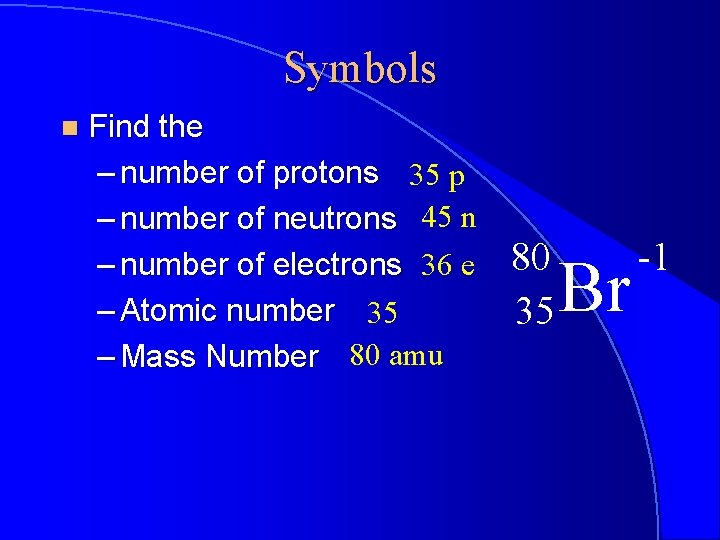
Symbols n Find the – number of protons 35 p – number of neutrons 45 n – number of electrons 36 e – Atomic number 35 – Mass Number 80 amu 80 35 Br -1

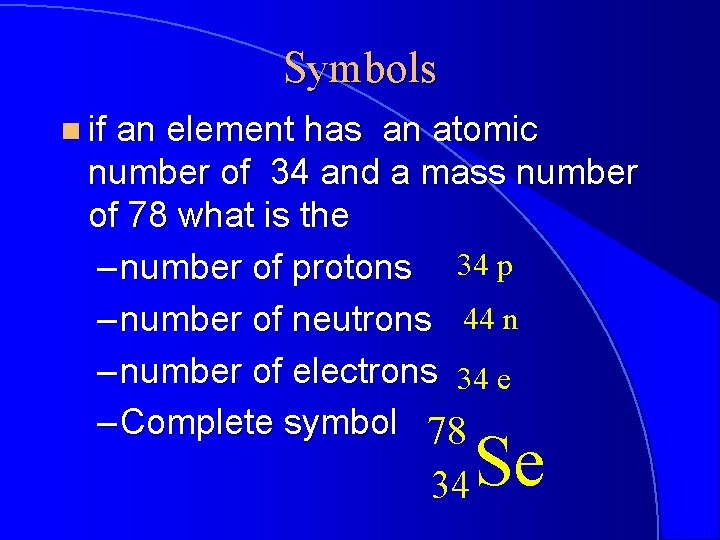
Symbols n if an element has an atomic number of 34 and a mass number of 78 what is the – number of protons 34 p – number of neutrons 44 n – number of electrons 34 e – Complete symbol 78 34 Se

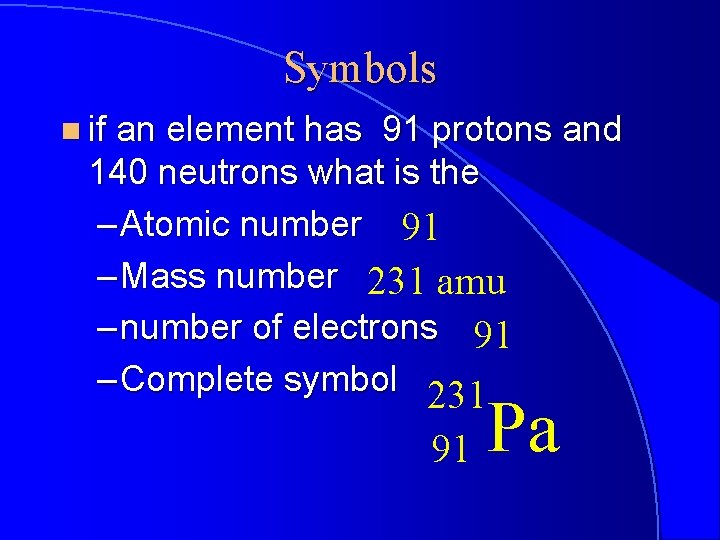
Symbols n if an element has 91 protons and 140 neutrons what is the – Atomic number 91 – Mass number 231 amu – number of electrons 91 – Complete symbol 231 91 Pa

Summary Mass number Element charge 1 1 Atomic number H +1 Element symbol

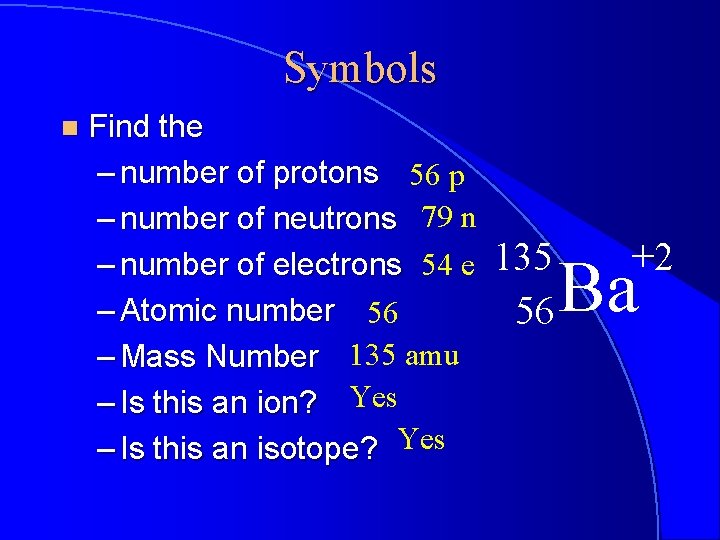
Symbols n Find the – number of protons 56 p – number of neutrons 79 n – number of electrons 54 e – Atomic number 56 – Mass Number 135 amu – Is this an ion? Yes – Is this an isotope? Yes 135 56 +2 Ba