Electron Configuration Electron Configuration Electron configuration is a






- Slides: 13

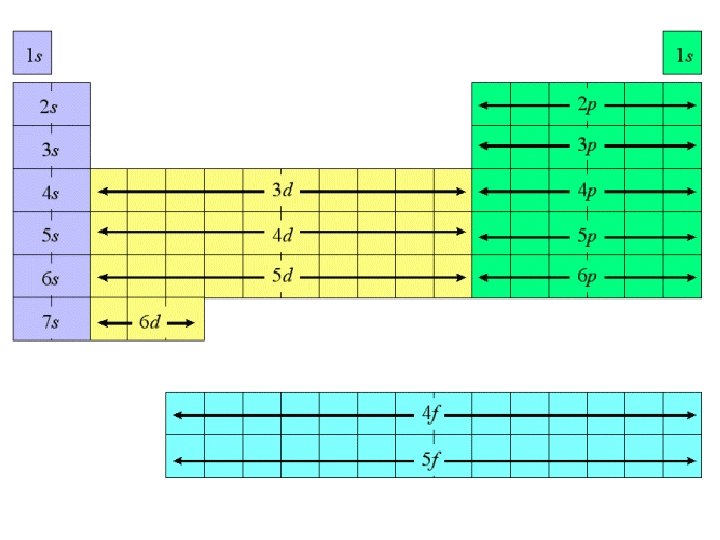
Electron Configuration


Electron Configuration • • Electron configuration is a shorthand notation that shows electron arrangement within orbitals. Electron configuration can be written in one of 3 methods: 1. Energy-Level Diagrams (orbital diagram) 2. Complete electron configuration 3. Condensed electron configuration (AKA noble gas configuration)

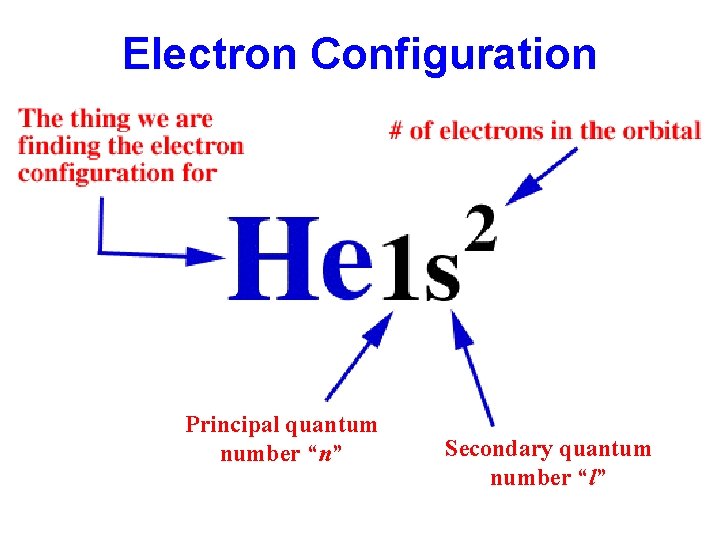
Electron Configuration Principal quantum number “n” Secondary quantum number “l”

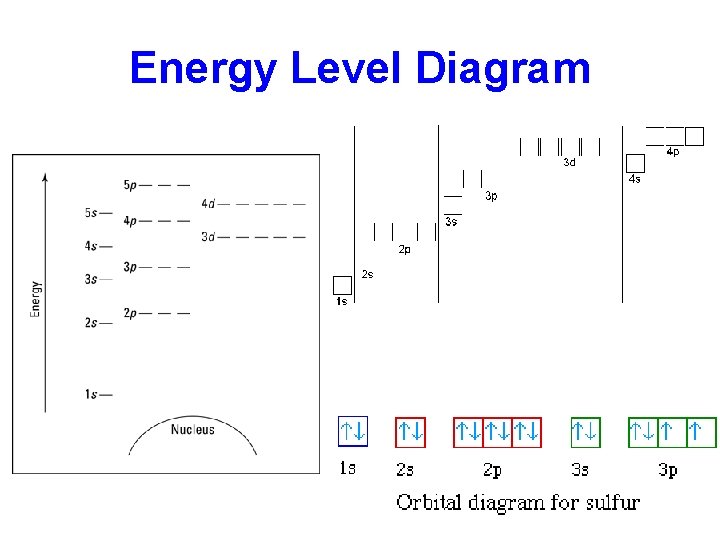
Energy Level Diagram

Pauli’s Exclusion Principle • No two electron’s have the same 4 quantum numbers • One electron will spin up, the other will spin down • Conventionally we write the electron that spins up first.

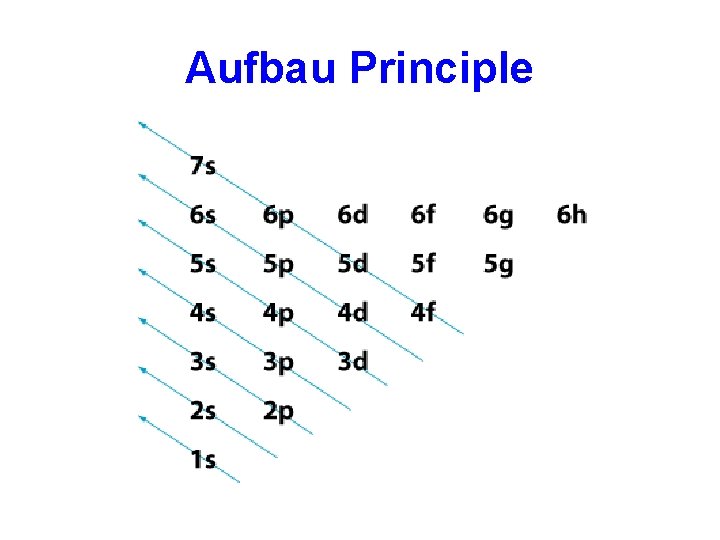
Aufbau Principle • The number of electrons in an atom is equal to the atomic number • Each added electron will enter the orbitals in the order of increasing energy • Orbitals of lowest energy are filled first • An orbital cannot take more than 2 electrons. • Analogy: You’re very tired after a night out. It’s easier to sleep downstairs on the couch than to climb the stairs to your bedroom. If your friend is already sleeping on the couch, then you will go upstairs.

Aufbau Principle

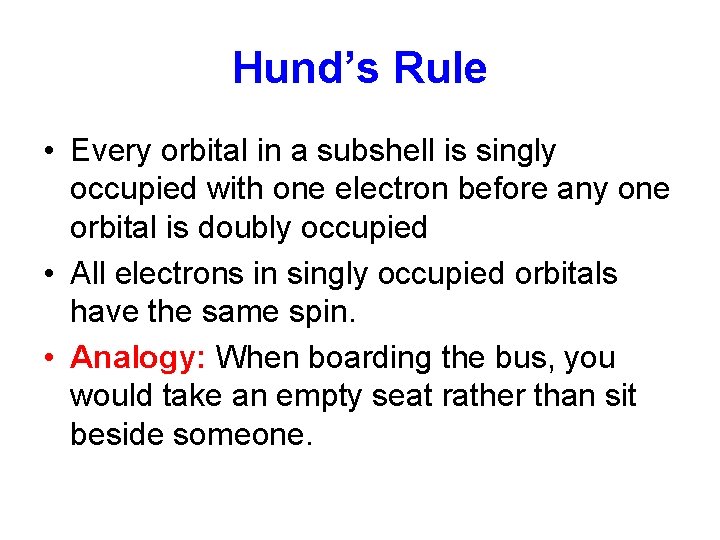
Hund’s Rule • Every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied • All electrons in singly occupied orbitals have the same spin. • Analogy: When boarding the bus, you would take an empty seat rather than sit beside someone.

• Lithium • Nitrogen • Manganese Examples

Electron Configuration for Ions • For anions: add extra electrons • For cations: draw the neutral atom, then subtract the required number of electrons from the orbital with the highest principal quantum number “n” • Examples S 2 -, Na+, Zn 2+

Practice • Activity • P. 191 #1 -5 • P. 194 #6 -11

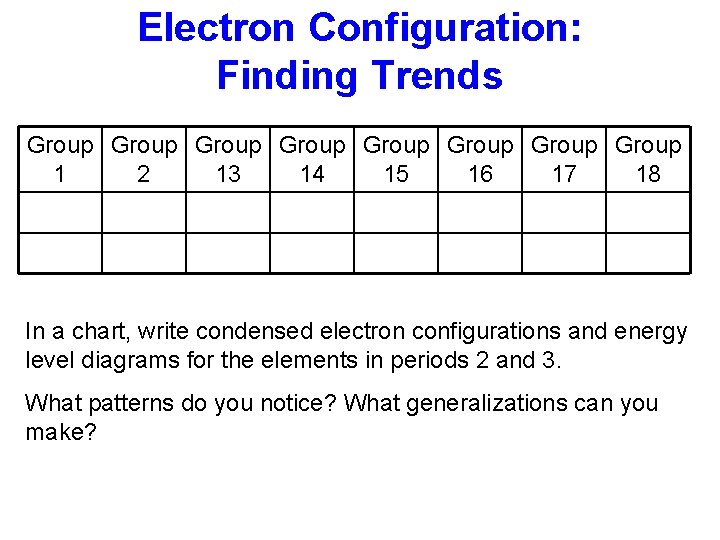
Electron Configuration: Finding Trends Group Group 1 2 13 14 15 16 17 18 In a chart, write condensed electron configurations and energy level diagrams for the elements in periods 2 and 3. What patterns do you notice? What generalizations can you make?