Electrons in Atoms Electron configuration Electrons z The







![Notation z. Shorthand Configuration S 16 e [Ne] Noble Gas 2 4 3 s Notation z. Shorthand Configuration S 16 e [Ne] Noble Gas 2 4 3 s](https://slidetodoc.com/presentation_image_h/343047a63bb05c1d31d4cb59ecbe5940/image-19.jpg)

- Slides: 22

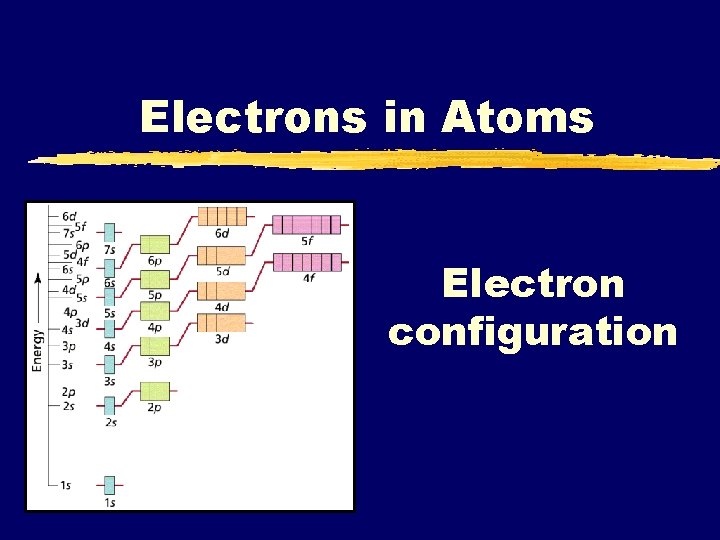
Electrons in Atoms Electron configuration

Electrons z. The arrangement of electrons in an atom will determine the chemical and physical properties of that atom (or ion). z. Because of this it is important to know where those electrons are (or are most likely to be found)

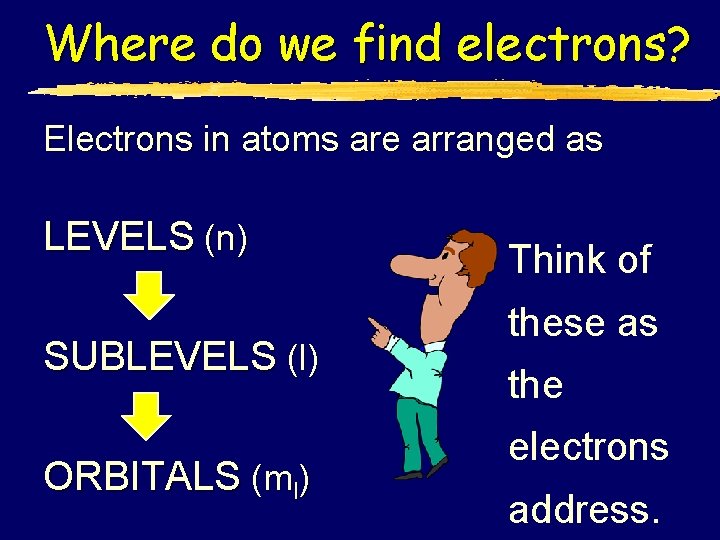
Where do we find electrons? Electrons in atoms are arranged as LEVELS (n) SUBLEVELS (l) ORBITALS (ml) Think of these as the electrons address.

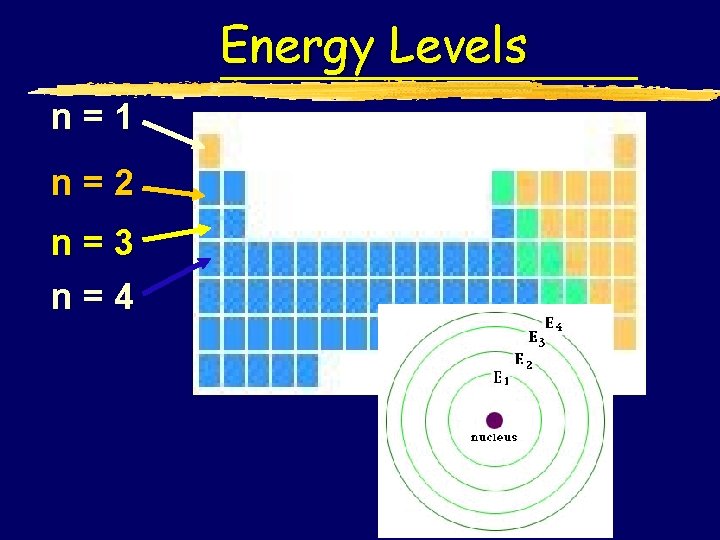
Energy Level (n) This tells you how far the electron is from the nucleus, the higher the energy level the further from the nucleus z. Currently n can be 1 thru 7, because there are 7 periods on the periodic table

Energy Levels n=1 n=2 n=3 n=4

Sublevels zattempt to describe where the electrons are likely to be found ys, p, d, f ORBITALS z. Found within the sublevels Each orbital can hold a maximum of 2 electrons

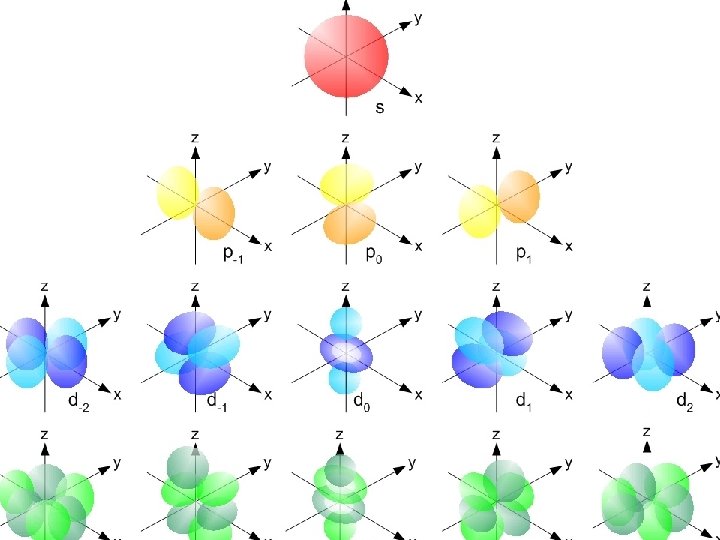
Types of Orbitals z. The most probable area to find these electrons takes on a shape z. So far, we have 4 shapes. They are named s, p, d, and f. z. Each orbital has different “flavors” ys= 1 flavor yp = 3 flavors yd = 5 flavors yf = 7 flavors x. Each “flavor” can hold a maximum of 2 electrons



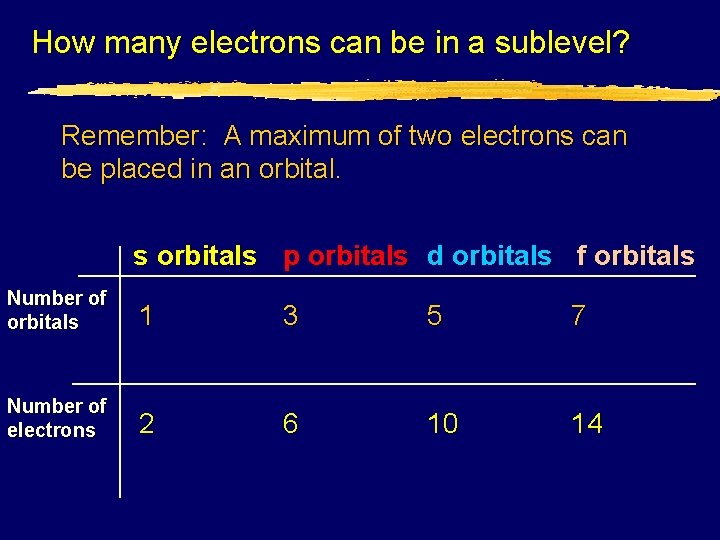
How many electrons can be in a sublevel? Remember: A maximum of two electrons can be placed in an orbital. s orbitals p orbitals d orbitals f orbitals Number of orbitals 1 3 5 7 Number of electrons 2 6 10 14

Electron possible per energy level 2 n 2 = the number of electrons possible in an energy level z. Ex: 3 rd energy level, n=3 2(3)2 = 18

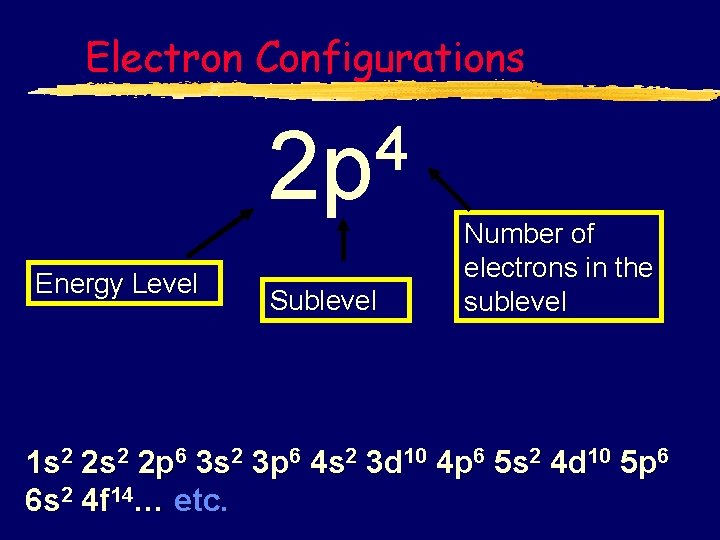
Electron Configurations 4 2 p Energy Level Sublevel Number of electrons in the sublevel 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 10 4 p 6 5 s 2 4 d 10 5 p 6 6 s 2 4 f 14… etc.

General Rules z. Pauli Exclusion Principle y. Each orbital can hold TWO electrons with opposite spins.

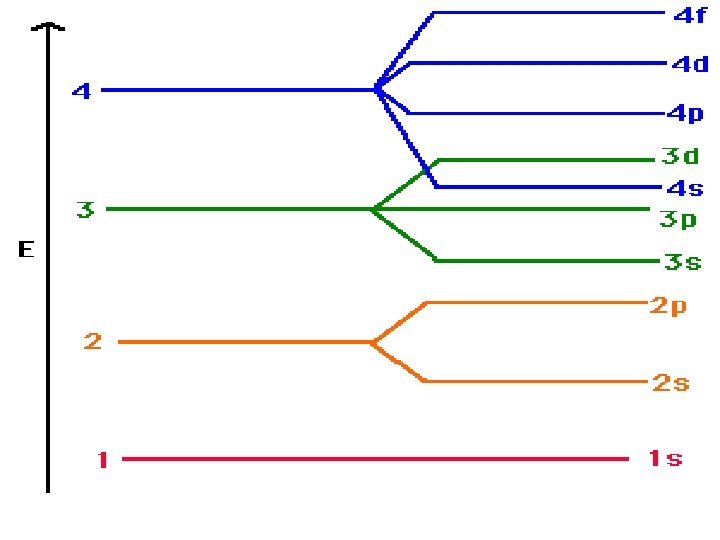
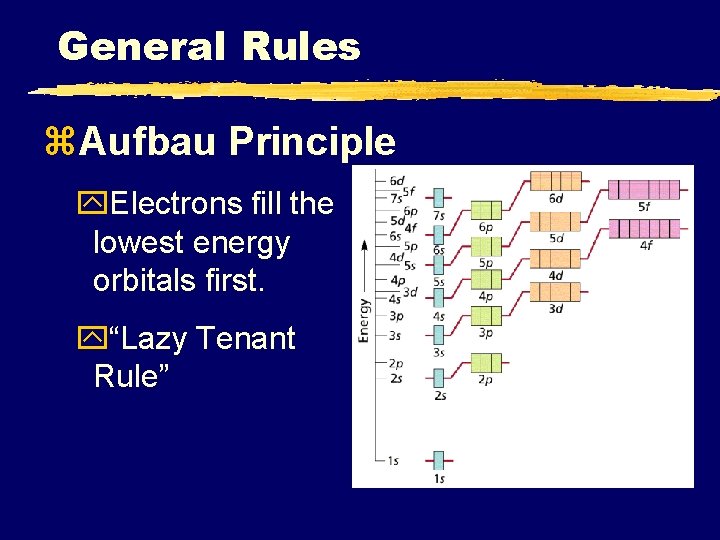
General Rules z. Aufbau Principle y. Electrons fill the lowest energy orbitals first. y“Lazy Tenant Rule”

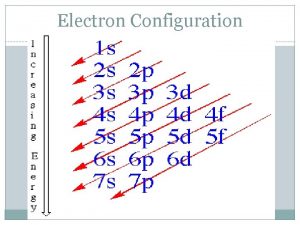
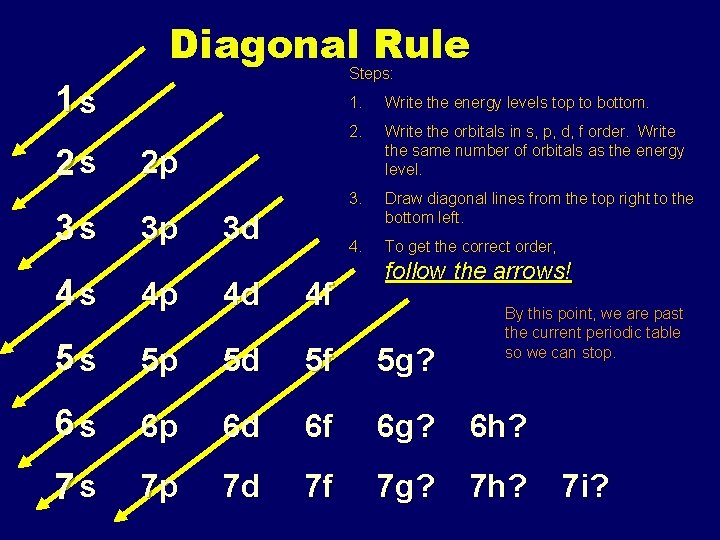
Diagonal Rule Steps: 1 s 2 s 3 s 1. Write the energy levels top to bottom. 2. Write the orbitals in s, p, d, f order. Write the same number of orbitals as the energy level. 3. Draw diagonal lines from the top right to the bottom left. 4. To get the correct order, 2 p 3 p 3 d follow the arrows! 4 s 4 p 4 d 4 f 5 s 5 p 5 d 5 f 5 g? 6 s 6 p 6 d 6 f 6 g? 6 h? 7 s 7 p 7 d 7 f 7 g? 7 h? By this point, we are past the current periodic table so we can stop. 7 i?

General Rules z. Hund’s Rule y. Within a sublevel, place one e- per orbital before pairing them. y“Empty Bus Seat Rule” WRONG RIGHT

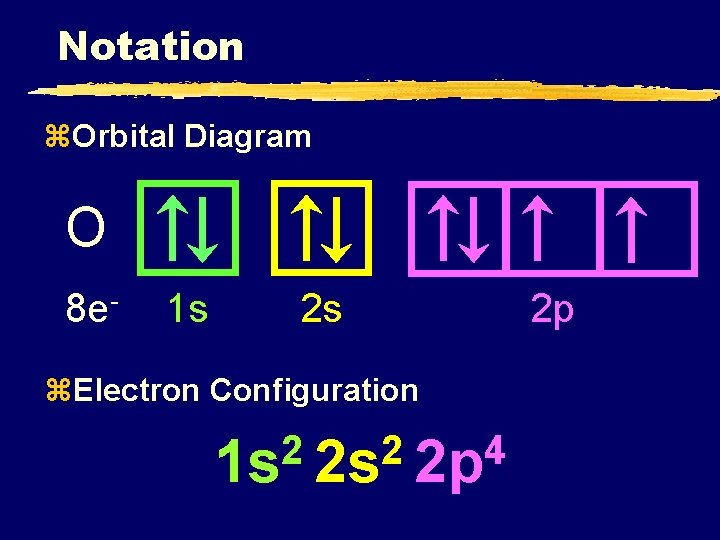
Notation z. Orbital Diagram O 8 e- 1 s 2 s z. Electron Configuration 2 2 4 1 s 2 s 2 p 2 p

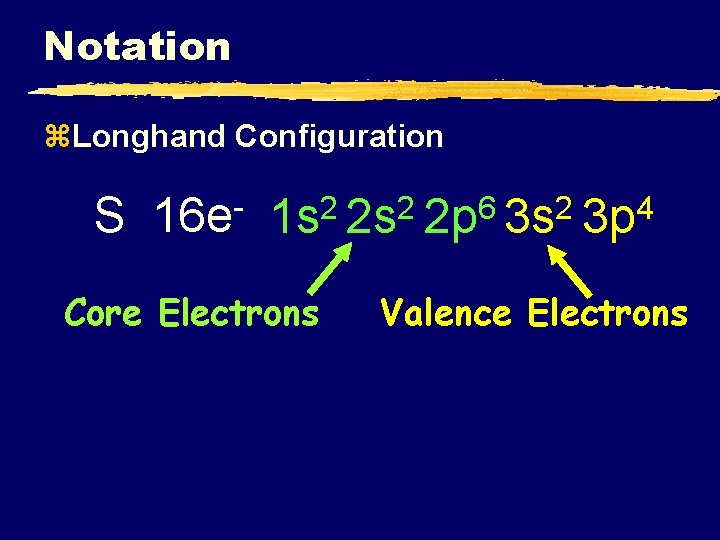
Notation z. Longhand Configuration S 16 e 6 2 2 2 1 s 2 s 2 p 3 s Core Electrons 4 3 p Valence Electrons
![Notation z Shorthand Configuration S 16 e Ne Noble Gas 2 4 3 s Notation z. Shorthand Configuration S 16 e [Ne] Noble Gas 2 4 3 s](https://slidetodoc.com/presentation_image_h/343047a63bb05c1d31d4cb59ecbe5940/image-19.jpg)
Notation z. Shorthand Configuration S 16 e [Ne] Noble Gas 2 4 3 s 3 p Valence Electrons

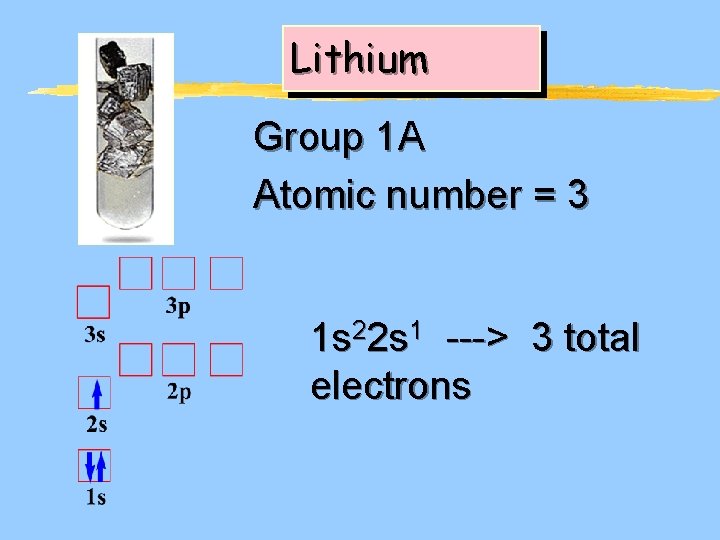
Lithium Group 1 A Atomic number = 3 1 s 22 s 1 ---> 3 total electrons

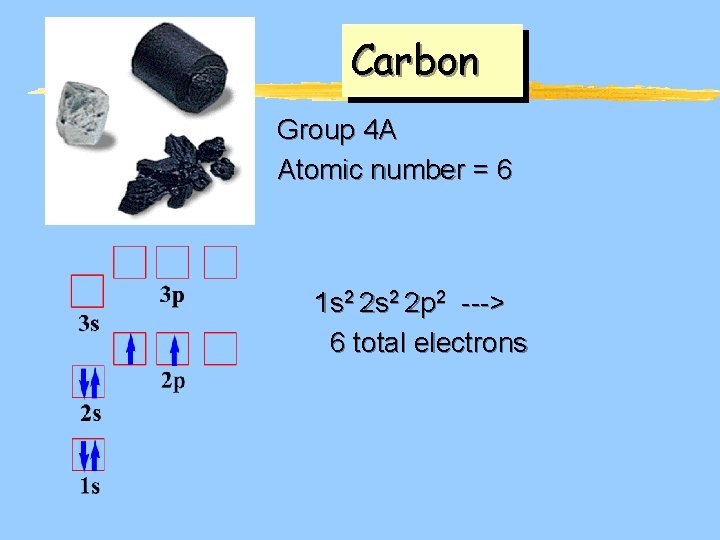
Carbon Group 4 A Atomic number = 6 1 s 2 2 p 2 ---> 6 total electrons

http: //www. youtube. com/watch? v=Vb 6 k. Axw. SWg. U
 Copper subshell configuration
Copper subshell configuration Electron configuration vs noble gas configuration
Electron configuration vs noble gas configuration Unpaired electrons
Unpaired electrons How to determine valence electrons
How to determine valence electrons Electron configuration for oxygen
Electron configuration for oxygen Regents periodic table
Regents periodic table How do chemists model the valence electrons of metal atoms?
How do chemists model the valence electrons of metal atoms? How to find the neutrons of an element
How to find the neutrons of an element Chapter 4 arrangement of electrons in atoms
Chapter 4 arrangement of electrons in atoms Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Ionic and metallic bonding chapter 7 practice problems
Ionic and metallic bonding chapter 7 practice problems Electrons in atoms section 1 light and quantized energy
Electrons in atoms section 1 light and quantized energy Chapter 5 review arrangement of electrons in atoms
Chapter 5 review arrangement of electrons in atoms Ccechs
Ccechs Atoms with 4 valence electrons
Atoms with 4 valence electrons 5 electrons in atoms
5 electrons in atoms Lowest allowable energy state of an atom
Lowest allowable energy state of an atom Atoms tend to gain lose or share electrons
Atoms tend to gain lose or share electrons Atoms with unpaired electrons are called diamagnetic.
Atoms with unpaired electrons are called diamagnetic. Electrons in atoms section 2 quantum theory and the atom
Electrons in atoms section 2 quantum theory and the atom Planar chirality
Planar chirality What is relative configuration
What is relative configuration Relative configuration vs absolute configuration
Relative configuration vs absolute configuration