Ionic Bonding Electron Configurations a Review and More

Ionic Bonding

Electron Configurations – a Review and More…

Keeping Track of Electrons • The electrons responsible for the chemical properties of atoms are those in the outer energy level. • Valence electrons - The s and p electrons in the outer energy level. • Core electrons -those in the energy levels below. • Basis for shorthand
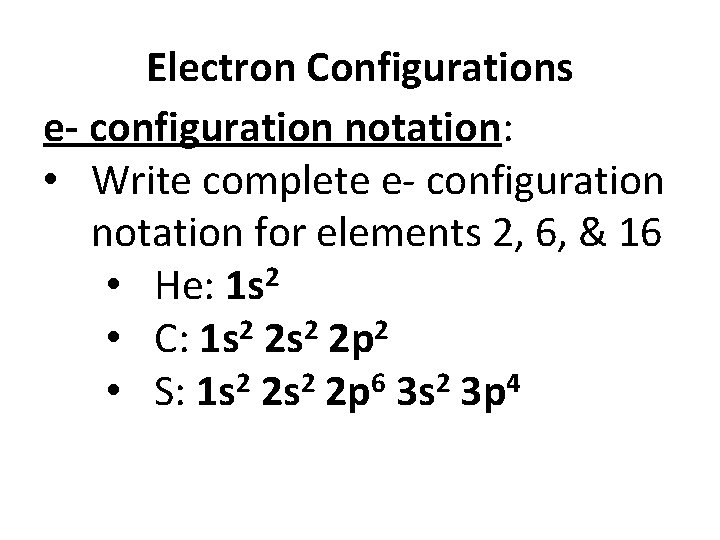
Electron Configurations e- configuration notation: • Write complete e- configuration notation for elements 2, 6, & 16 • He: 1 s 2 • C: 1 s 2 2 p 2 2 2 6 2 4 • S: 1 s 2 s 2 p 3 s 3 p

Electron Configurations e- configuration notation (noble gas shortcut): • Reminder – this version uses a noble gas (group 18) “core” instead of beginning at 1 s
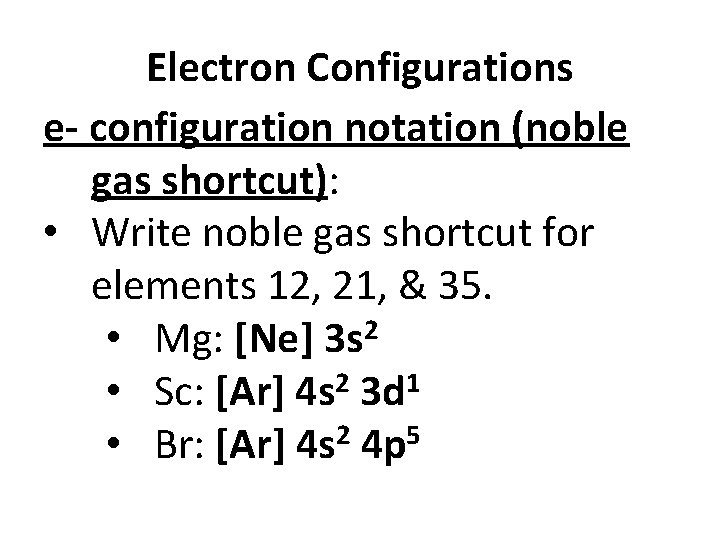
Electron Configurations e- configuration notation (noble gas shortcut): • Write noble gas shortcut for elements 12, 21, & 35. • Mg: [Ne] 3 s 2 2 1 • Sc: [Ar] 4 s 3 d • Br: [Ar] 4 s 2 4 p 5

Lewis dot diagrams lewis dot notation: • simplest notation, only shows valence e- (e- that may be lost, gained, or shared when chemical compounds are formed - they are from s & p blocks)

Lewis Dot Lewis dot notation: • Draw dot diagrams for elements 1 -10

Ion Formation…

Ion Formation… Valence electrons: • outer shell electrons that may be lost, gained, or shared when chemical compounds are formed
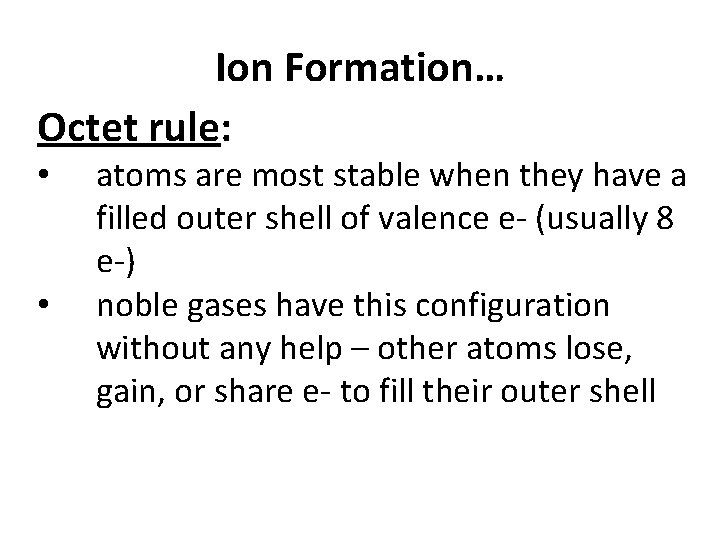
Ion Formation… Octet rule: • • atoms are most stable when they have a filled outer shell of valence e- (usually 8 e-) noble gases have this configuration without any help – other atoms lose, gain, or share e- to fill their outer shell

Ion Formation… Ions: • Atoms that have either gained or lost e-. –Gain of e- gives a negative ion called an anion. –Loss of e gives a positive ion called a cation.

Ion Formation… Ion examples: • The magnesium ion is Mg 2+. How many p+ and e- does it have? 12 + p, 10 e

Ion Formation… Ion examples: • The oxide ion is O 2 -. How many p+ and e- does it have? 8 + p, 10 e

Ion Formation… Ion examples: • An ion has 7 p+ and 10 e-. What ion is it? 3 N

Ion Formation… Ion examples: • An ion has 4 p+ and 2 e-. What ion is it? 2+ Be

Keeping Track of Electrons • • • Atoms in the same column Have the same properties because Have the same outer electron configuration. Have the same valence electrons. Group 2 - Be, Mg, Ca, etc. 2 valence electrons
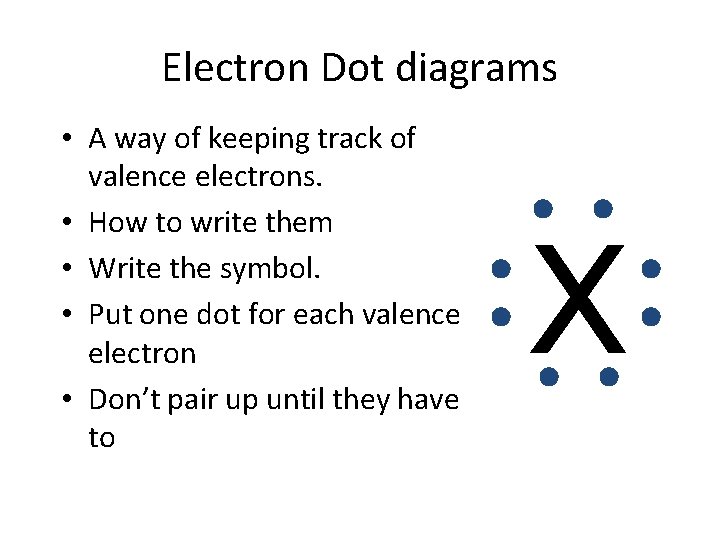
Electron Dot diagrams • A way of keeping track of valence electrons. • How to write them • Write the symbol. • Put one dot for each valence electron • Don’t pair up until they have to X

The Electron Dot diagram for Nitrogen § Nitrogen has 5 valence electrons. § First we write the symbol. §Then add 1 electron at a time to each side. §Until they are forced to pair up. N
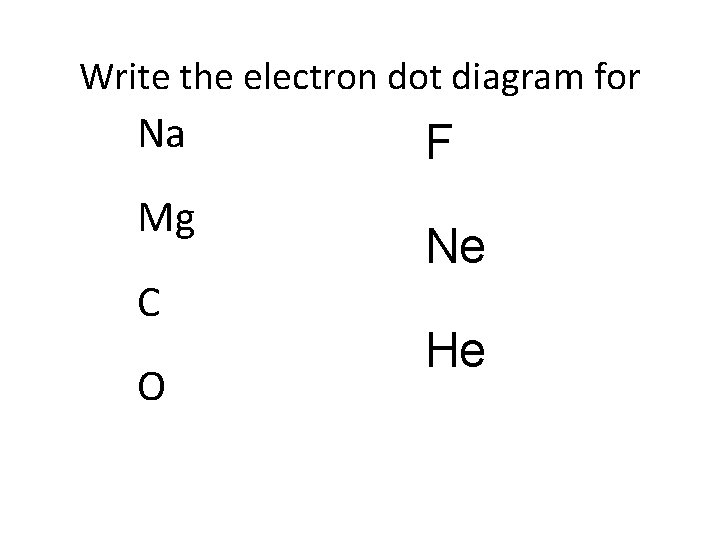
Write the electron dot diagram for Na Mg C O F Ne He

Electron Configurations for Cations • Metals lose electrons to attain noble gas configuration. • They make positive ions. • Na 1 s 22 p 63 s 1 - 1 valence electron • Na+ 1 s 22 p 6 -noble gas configuration

Electron Dots For Cations • Metals will have few valence electrons Ca

Electron Dots For Cations • Metals will have few valence electrons • These will come off Ca

Electron Dots For Cations • Metals will have few valence electrons • These will come off • Forming positive ions 2+ Ca
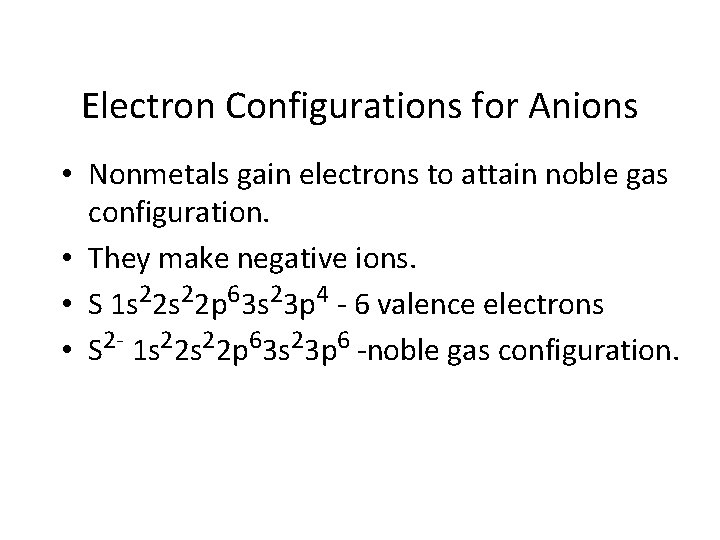
Electron Configurations for Anions • Nonmetals gain electrons to attain noble gas configuration. • They make negative ions. • S 1 s 22 p 63 s 23 p 4 - 6 valence electrons • S 2 - 1 s 22 p 63 s 23 p 6 -noble gas configuration.
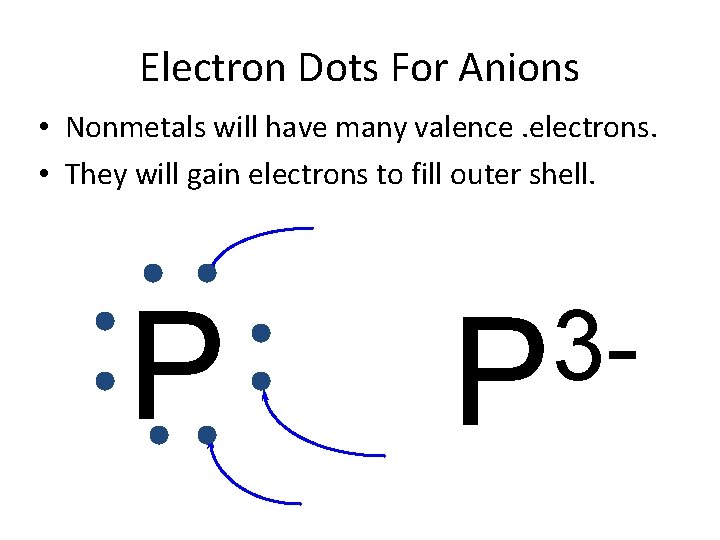
Electron Dots For Anions • Nonmetals will have many valence. electrons. • They will gain electrons to fill outer shell. P 3 P
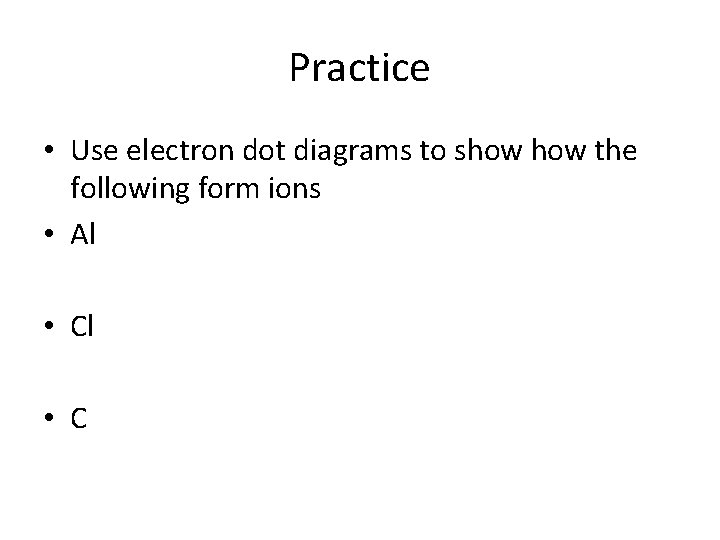
Practice • Use electron dot diagrams to show the following form ions • Al • C
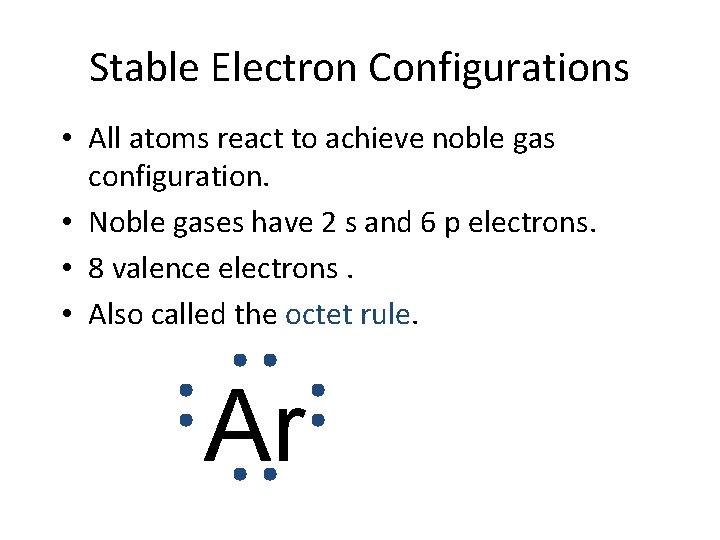
Stable Electron Configurations • All atoms react to achieve noble gas configuration. • Noble gases have 2 s and 6 p electrons. • 8 valence electrons. • Also called the octet rule. Ar

Transition metals • • • Form cations Hard to predict the charge Often will form more than 1 charge Can’t form noble gas configuration Still try to fill up orbitals Some can make pseudo noble gas configurations with full orbitals

Polyatomic ions • Groups of atoms that stick together as a unit, and have a charge • (PO 4)3 - phosphate • (CO 3)2 - carbonate • (NH 4)+ Ammonium • (NO 3) 1 - Nitrate • (NO 2) 1 - Nitrite • Names often end in –ate or –ite

Ionic Bonding • Anions and cations are held together by opposite charges. • This is the bond • Ionic compounds are called salts. • Simplest ratio is called the formula unit. • The bond is formed through the transfer of electrons. • Electrons are transferred to achieve noble gas configuration.

Ionic Bonding 1+ 1 - Na Cl
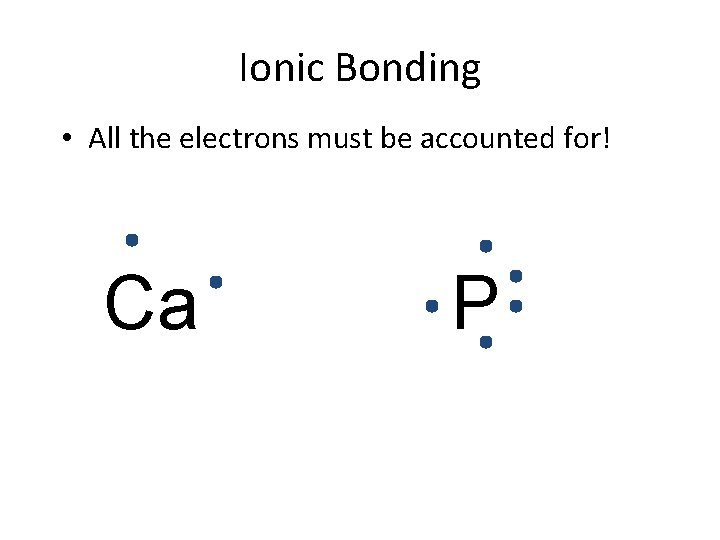
Ionic Bonding • All the electrons must be accounted for! Ca P
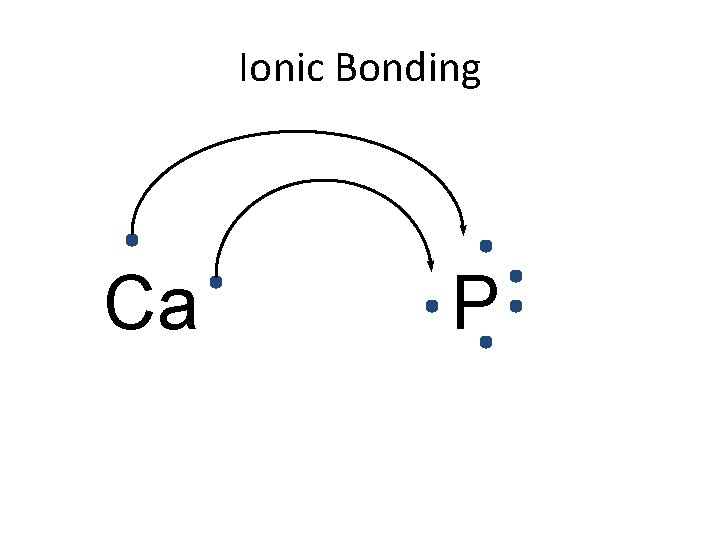
Ionic Bonding Ca P
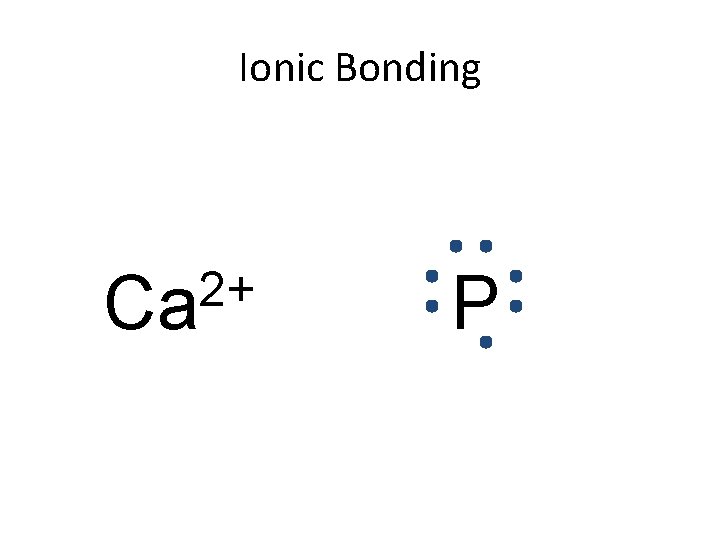
Ionic Bonding 2+ Ca P
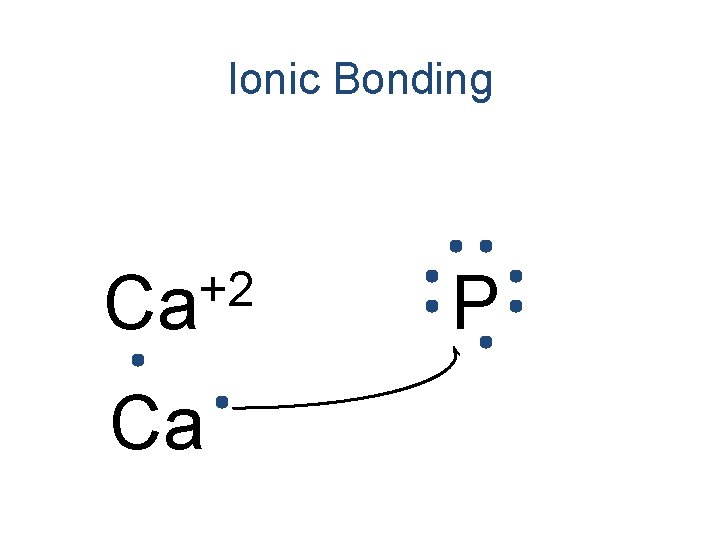
Ionic Bonding +2 Ca Ca P
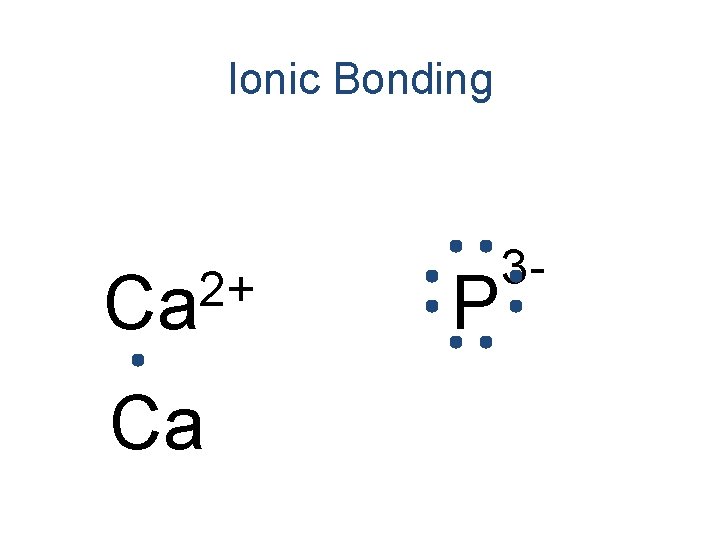
Ionic Bonding 2+ Ca Ca P 3 -
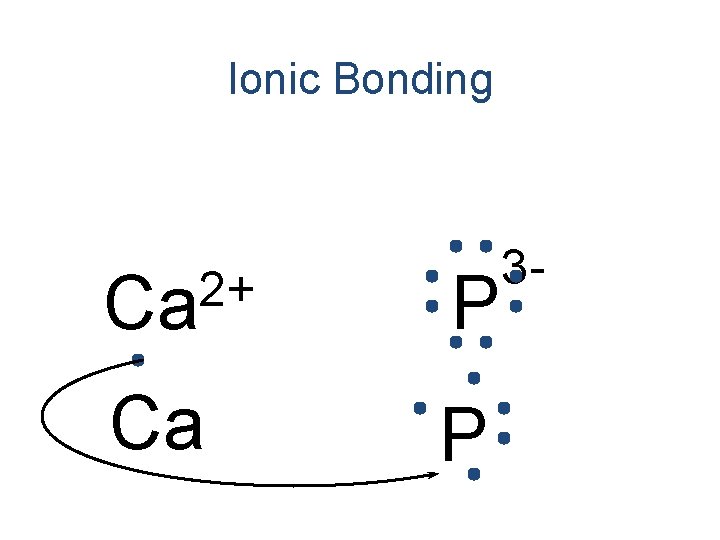
Ionic Bonding 2+ Ca P 3 -
Ionic Bonding 2+ Ca P 3 -

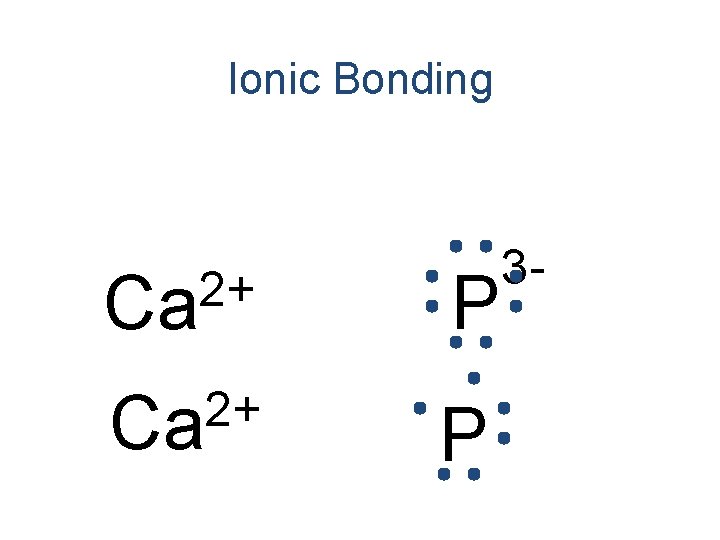
Ionic Bonding Ca 2+ Ca P 3 -
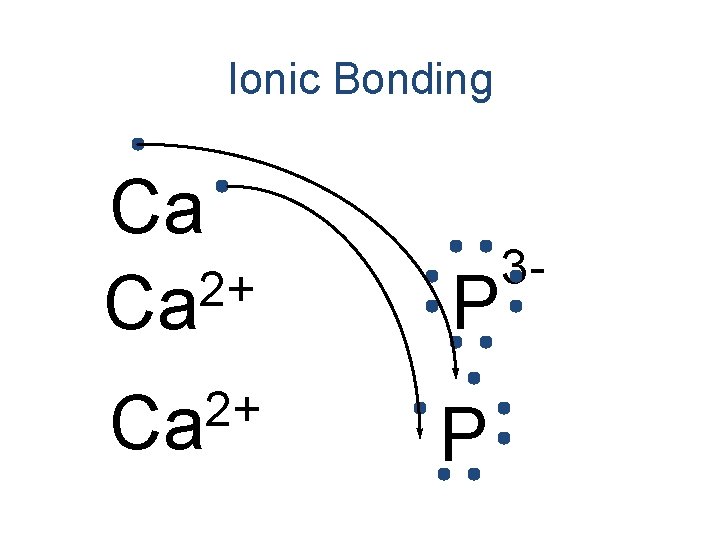
Ionic Bonding Ca 2+ Ca P 3 -

Ionic Bonding 2+ Ca P P 3 - 3 -

Ionic Bonding Ca 3 P 2 Formula Unit
Practice • Use electron dot diagrams to show the following elements make an ionic compound and write the formula unit • Mg and Cl

Practice • Na and N

Practice • Al and O

Writing formulas • The charges must add to 0 • Add the correct subscript to make them equal zero • Na 1+ O 2 • Sr 2+ Cl 1 • Fe 3+ O 2 • Potassium bromide • Beryllium fluoride

Polyatomic ionic compounds (NH 4) + and N 3 – It will take three (NH 4) + to bond with One N 3 – You must have a neutral compound in ALL cases! So we write (NH 4)3 N This says 3 ammonium ions bonded to one Nitride ion

Ionic Compounds • Made up of – a positive and negative ion – a cation and an anion – a metal and a nonmetal • Smallest repeating unit- formula unit
Properties of Ionic Compounds • Crystalline structure. • A regular repeating arrangement of ions in the solid. • Ions are strongly bonded. • Structure is rigid. • High melting points- because of strong forces between ions.
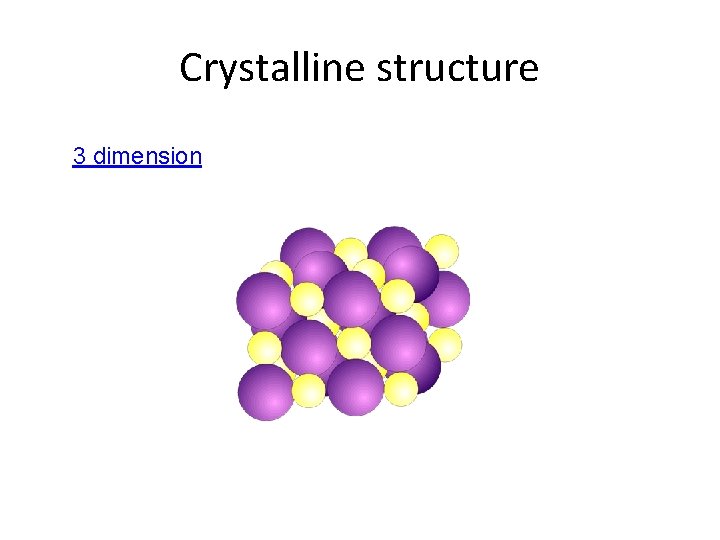
Crystalline structure 3 dimension
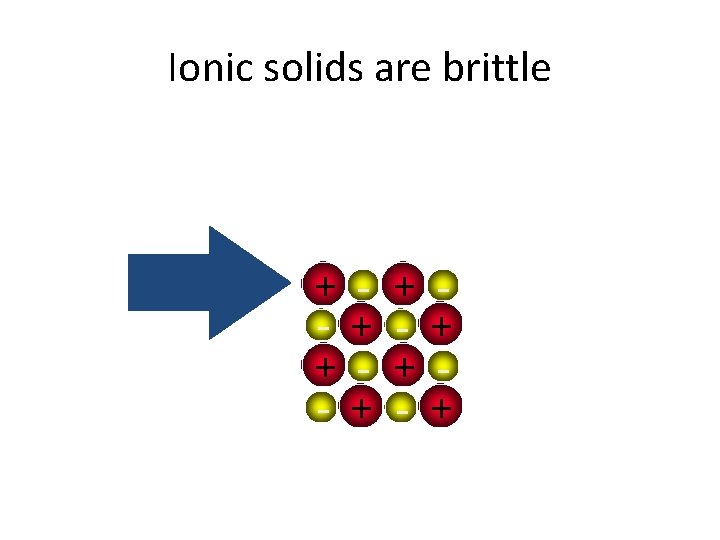
Ionic solids are brittle + + - + +
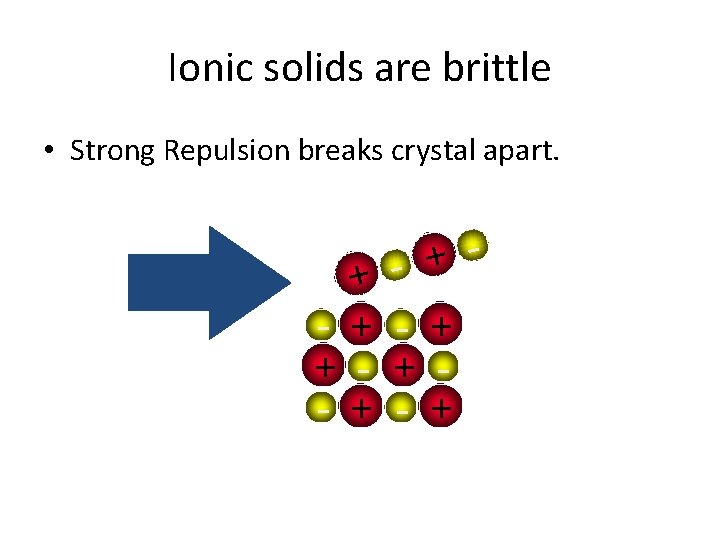
Ionic solids are brittle • Strong Repulsion breaks crystal apart. + + - + - + - +
Crystal Structures • The repeating unit is called the unit cell

Do they Conduct? • Conducting electricity is allowing charges to move. • In a solid, the ions are locked in place. • Ionic solids are insulators. • When melted, the ions can move around. • Melted ionic compounds conduct. • First get them to 800ºC. • Dissolved in water they conduct.
- Slides: 55