GENERAL CHEMISTRY CHEM 110 REVISION CHAPTER 7 Energy
![[1] Calculate the energy, in joules, required to excite a hydrogen atom by causing [1] Calculate the energy, in joules, required to excite a hydrogen atom by causing](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-6.jpg)
![[3] What is the four quantum numbers for the last electron in Aluminum(Al 13) [3] What is the four quantum numbers for the last electron in Aluminum(Al 13)](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-7.jpg)
![[6] Which one of the following electrons have the same energy as an electron [6] Which one of the following electrons have the same energy as an electron](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-8.jpg)
![[9] Which statement about the four quantum numbers which describe electrons in atoms is [9] Which statement about the four quantum numbers which describe electrons in atoms is](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-9.jpg)
![[12] A possible set of quantum numbers for the last electron added to complete [12] A possible set of quantum numbers for the last electron added to complete](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-10.jpg)
![[15] Which one of the following sets of quantum number is not possible? A. [15] Which one of the following sets of quantum number is not possible? A.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-11.jpg)
![[18] Choose the incorrect quantum numbers for electron in an atom? A. (3 [18] Choose the incorrect quantum numbers for electron in an atom? A. (3](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-12.jpg)
![[21] The maximum number of electron for one orbital is? A. 2 electron B. [21] The maximum number of electron for one orbital is? A. 2 electron B.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-13.jpg)
![[24] Atoms of which two elements have the same number of unpaired electrons? A. [24] Atoms of which two elements have the same number of unpaired electrons? A.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-14.jpg)
![[27]The ground-state electron configuration for an atom of indium (In) is A. B. C. [27]The ground-state electron configuration for an atom of indium (In) is A. B. C.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-15.jpg)
![[30] Atoms of which two elements have the same number of unpaired electrons? A. [30] Atoms of which two elements have the same number of unpaired electrons? A.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-16.jpg)
![[33] Which of the following is paramagnetic? (A) K+ (B) Sn+2 (C) Sc+3 (D) [33] Which of the following is paramagnetic? (A) K+ (B) Sn+2 (C) Sc+3 (D)](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-17.jpg)
![[36] A ground –state atom of Mn+2 has -----unpaired electrons and is -----. A. [36] A ground –state atom of Mn+2 has -----unpaired electrons and is -----. A.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-18.jpg)
![[38] Which two electron configurations represent elements that would have similar chemical properties? 1. [38] Which two electron configurations represent elements that would have similar chemical properties? 1.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-19.jpg)
![[40] Which of the orbital diagrams is not follow the pauli exclusion principle? ↑ [40] Which of the orbital diagrams is not follow the pauli exclusion principle? ↑](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-20.jpg)
![[42]Choose the incorrect quantum numbers for electron in an atom? A. (3 , [42]Choose the incorrect quantum numbers for electron in an atom? A. (3 ,](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-21.jpg)
![[45] The electron configuration of a neutral atom is[Ne] 3 s 23 p 3. [45] The electron configuration of a neutral atom is[Ne] 3 s 23 p 3.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-22.jpg)


- Slides: 25

GENERAL CHEMISTRY CHEM 110 REVISION CHAPTER 7

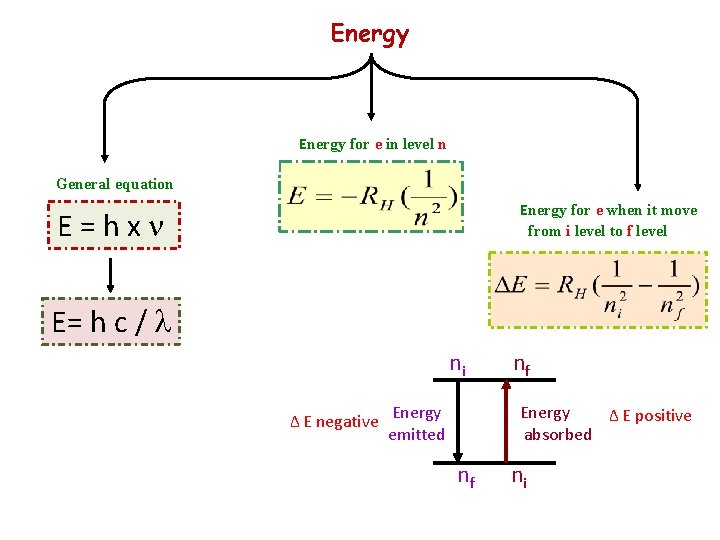
Energy for e in level n General equation Energy for e when it move from i level to f level E = h x n E= h c / l ni ∆ E negative Energy emitted nf Energy ∆ E positive absorbed nf ni

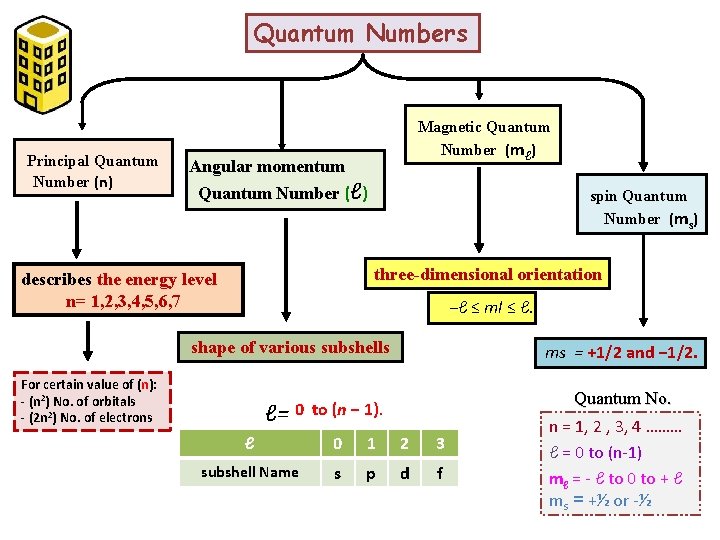
Quantum Numbers Principal Quantum Number (n) Magnetic Quantum Number (mℓ) Angular momentum Quantum Number (ℓ) spin Quantum Number (ms) three-dimensional orientation describes the energy level n= 1, 2, 3, 4, 5, 6, 7 −ℓ ≤ ml ≤ ℓ. shape of various subshells For certain value of (n): - (n 2) No. of orbitals - (2 n 2) No. of electrons ℓ= ms = +1/2 and − 1/2. Quantum No. 0 to (n − 1). ℓ 0 1 2 3 subshell Name s p d f n = 1, 2 , 3, 4 ……… ℓ = 0 to (n-1) mℓ = - ℓ to 0 to + ℓ ms = +½ or -½

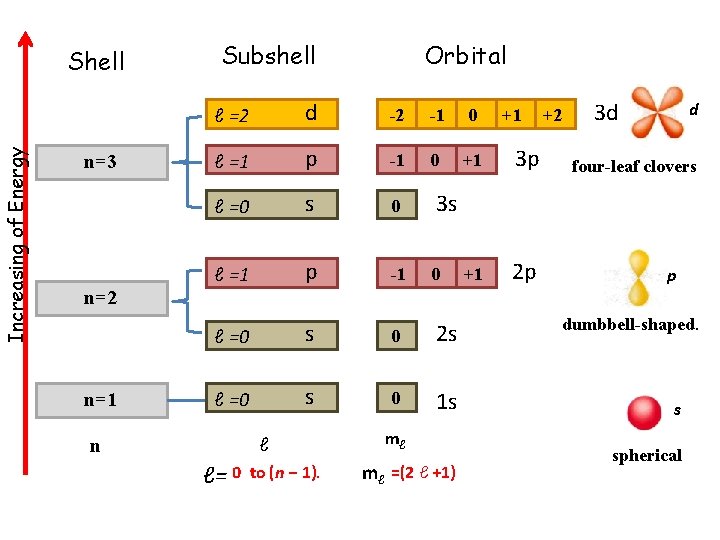
Increasing of Energy Shell n=3 Subshell Orbital ℓ =2 d -2 -1 0 ℓ =1 p -1 0 +1 3 p ℓ =0 s 0 3 s ℓ =1 p -1 0 +1 2 p ℓ =0 s 0 2 s ℓ =0 s 0 1 s +1 +2 3 d d four-leaf clovers p n=2 n=1 ℓ n ℓ= 0 to (n − 1). mℓ mℓ =(2 ℓ +1) dumbbell-shaped. s spherical

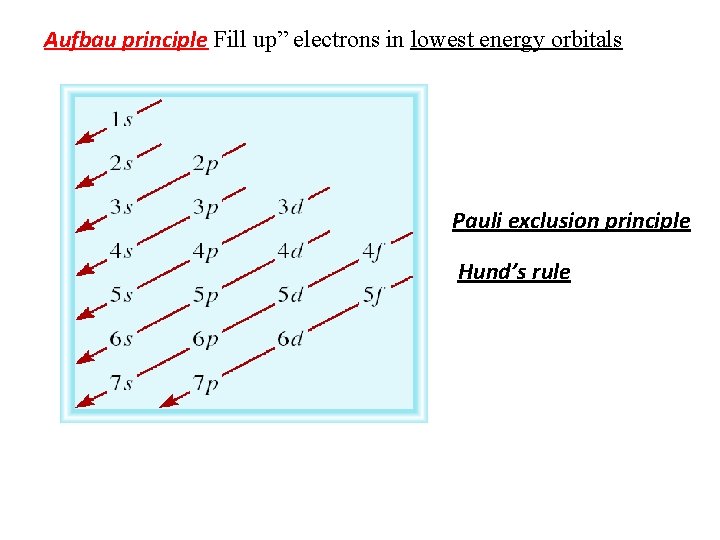
Aufbau principle Fill up” electrons in lowest energy orbitals Pauli exclusion principle Hund’s rule
![1 Calculate the energy in joules required to excite a hydrogen atom by causing [1] Calculate the energy, in joules, required to excite a hydrogen atom by causing](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-6.jpg)
[1] Calculate the energy, in joules, required to excite a hydrogen atom by causing an electronic transition from the n = 1 to the n = 4 principal energy level. A. 2. 04 10 - 18 J B. 2. 19 105 J C. 2. 04 10 -29 J D. 3. 27 10 -17 J [2] What is the wavelength of radiation that has a frequency of 7. 5× 1014 s-1? A. 2. 3× 106 nm B. 4. 3× 10 -7 nm C. 2. 0× 1023 nm D. 4. 0× 102 nm
![3 What is the four quantum numbers for the last electron in AluminumAl 13 [3] What is the four quantum numbers for the last electron in Aluminum(Al 13)](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-7.jpg)
[3] What is the four quantum numbers for the last electron in Aluminum(Al 13) atom A. n=2, ℓ= 1, mℓ=-1, ms=+1/2 B. n=3, ℓ=3, mℓ=-1, ms=+1/2 C. n=3, ℓ=1, mℓ=-1, ms=+1/2 D. n=2, ℓ= 1, mℓ=-1, ms=-1/2 [4] Which one of the following electrons has the same energy as an electron with quantum numbers n = 5, l = 2, ml = -1 , ms = +1/2? A. n = 5, l = 2, ml = 0, ms = +1/2 B. n = 4, l = 3, ml = -1, ms = +1/2 C. n = 4, l = 2, ml = 3, ms = -1/2 D. None of these [5] What is the four quantum numbers for the last electron in Sodium (Na 11) atom A. B. C. D. n = 3, ℓ = 1, mℓ = 0, ms = -½ n = 3, ℓ = 0, ms =+ ½ n = 2, ℓ = 1, mℓ = -1, ms = +½ n = 2, ℓ = 0, mℓ = -1, ms =+ ½
![6 Which one of the following electrons have the same energy as an electron [6] Which one of the following electrons have the same energy as an electron](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-8.jpg)
[6] Which one of the following electrons have the same energy as an electron with quantum numbers n = 4, l = 3, ml = -1 , ms = +1/2? A. n = 5, l = 1, ml = -1 , ms = ½ B. n = 4, l = 3, ml = -1 , ms = ½ C. n = 4, l = 2, ml = 2 , ms = -½ D. None of these [7 ]Which one of the following electrons have the same energy as an electron with quantum numbers n = 4, l = 2, ml = -1 , ms = +1/2? A. n=5, l = 1, ml = -1, ms= +1/2 B. n=4, l = 3, ml = -1, ms= +1/2 C. n=4, l = 2, ms= -1/2 D. None of these [8] What is the four quantum numbers for the last electron in Silicon (Si) atom A. B. C. D. n= 3, l = 2, ml = 0, ms = - ½ n= 3, l = 1, ml = 0, ms = +½ n= 2, l = 1, ml = -1, ms = -½ n= 2, l = 0, ml = -1, ms = +½
![9 Which statement about the four quantum numbers which describe electrons in atoms is [9] Which statement about the four quantum numbers which describe electrons in atoms is](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-9.jpg)
[9] Which statement about the four quantum numbers which describe electrons in atoms is incorrect? A. n = principal quantum number, n = 1, 2, 3, . . . B. l = subsidiary quantum number, l = 1, 2, 3, . . . , (n+1) C. ml = magnetic quantum number, ml = (-l), . . , 0, . . , (+l) D. ms = spin quantum number, ms = +1/2 or -1/2. [10] What is the total number of orbitals in the n = 3 level? (A) 2 (B) 8 (C) 9 (D) 3 [11] Which of the following statements about the quantum numbers is incorrect? (A) n has integral values from 1 to ∞ (B) L has values from 1 to ∞ (C) m. L has values of -L to +L, including zero (D) ms has values of + ,
![12 A possible set of quantum numbers for the last electron added to complete [12] A possible set of quantum numbers for the last electron added to complete](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-10.jpg)
[12] A possible set of quantum numbers for the last electron added to complete an atom of gallium Ga in its ground state is n L ms (A) 4 0 -1/2 (B) 3 1 0 +1/2 (C) 4 1 +1/2 (D) 3 1 +1 -1/2 [13]The number of orbitals in a d subshell is? A. 1 B. 3 C. 5 D. 7 [14] The maximum number of electron for one orbital is? A. 2 electron B. 1 electron C. 3 electron D. 6 electron
![15 Which one of the following sets of quantum number is not possible A [15] Which one of the following sets of quantum number is not possible? A.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-11.jpg)
[15] Which one of the following sets of quantum number is not possible? A. (3 , 0 , +1/2 ) B. (4 , 3 , -2 , -1/2 ) C. (1 , 0 , +1/2 ) D. (3 , 2 , 1 ) [16]The last two electrons in C 6 atom should differ in the value of the quantum number: A. n B. l C. ml D. ms [17] The representative elements are those with unfilled energy levels in which the "last electron" was added to A. an s or p orbital. . B. an s orbital. C. a p or d orbital. D. an f orbital.
![18 Choose the incorrect quantum numbers for electron in an atom A 3 [18] Choose the incorrect quantum numbers for electron in an atom? A. (3](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-12.jpg)
[18] Choose the incorrect quantum numbers for electron in an atom? A. (3 , 0 , +1/2) B. (3 , 5 , 0 , +1/2) C. (1 , 0 , +1/2) D. (4 , 3 , -2 , -1/2) [19 The number of orbital in a p subshell is? A. 1 B. 7 C. 5 D. 3 [20] The maximum number of electrons that can occupy an energy level described by the principal quantum number, n, is? A. n+1 B. 2 n C. n 2 D. 2 n 2
![21 The maximum number of electron for one orbital is A 2 electron B [21] The maximum number of electron for one orbital is? A. 2 electron B.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-13.jpg)
[21] The maximum number of electron for one orbital is? A. 2 electron B. 3 electron C. 1 electron D. 6 electron [22] The electron configuration of a neutral atom is[Ne] 3 s 23 p 3. The four quantum numbers of the last electron are: A. (3 , 1 , +1/2 ) B. (2 , 0 , +1/2 ) C. (2 , 0 , -1 , +1/2 ) D. (3 , 2 , -1 , +1/2 ) [23] Atoms of which two elements have the same number of unpaired electrons? A. Na and C B. O and C C. Mg and C D. Al and C
![24 Atoms of which two elements have the same number of unpaired electrons A [24] Atoms of which two elements have the same number of unpaired electrons? A.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-14.jpg)
[24] Atoms of which two elements have the same number of unpaired electrons? A. Si and Cl B. Al and Cl C. Mg and Cl D. N and Cl [25] Which anion contains the most number of electrons? A. P 3 B. Se 2 C. Si 4 D. S 2[26] Atoms of which two elements have the same number of unpaired electrons? A. Si and F B. B and F C. Ca and F D. N and F
![27The groundstate electron configuration for an atom of indium In is A B C [27]The ground-state electron configuration for an atom of indium (In) is A. B. C.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-15.jpg)
[27]The ground-state electron configuration for an atom of indium (In) is A. B. C. D. [Ar]4 s 23 d 104 p 1. [Ar]4 s 24 p 63 d 5. [Kr]5 s 25 p 64 d 5. [Kr]5 s 24 d 105 p 1. [28] The [Ar]4 s 24 p 5 element are found in _______ of the periodic table. A. B. C. D. Group 2 A Group 7 A Period 7 Group 8 A [29] How many electrons are found in the (Sn 2+) ion? A. 48 B. 119 C. 22 D. 69
![30 Atoms of which two elements have the same number of unpaired electrons A [30] Atoms of which two elements have the same number of unpaired electrons? A.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-16.jpg)
[30] Atoms of which two elements have the same number of unpaired electrons? A. Na and S B. Si and S C. Mg and S D. Al and S [31] Which ground-state atom has an electron configuration described by the following orbital diagram? (A) phosphorus (B) germanium (C) selenium (D) tellurium [32] All the following electronic ground-state configurations are correct except (A) 20 Ca: [Ar] 4 s 2 (B) 25 Mn: [Ar] 4 s 2 4 d 5 (C) 29 Cu: [Ar] 4 s 13 d 10 (D) 54 Xe: [Kr] 5 s 24 d 10 5 p 6
![33 Which of the following is paramagnetic A K B Sn2 C Sc3 D [33] Which of the following is paramagnetic? (A) K+ (B) Sn+2 (C) Sc+3 (D)](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-17.jpg)
[33] Which of the following is paramagnetic? (A) K+ (B) Sn+2 (C) Sc+3 (D) Cr+3 [34] The general electron configuration for atoms of all elements in Group 5 A is (A) ns 2 np 1 (B) ns 2 np 5 (C) ns 2 np 4 (D) ns 2 np 3 [35] What is the electron configuration of the Cr 24? A. ( 1 s 2 2 p 6 3 s 2 3 p 5 4 s 2 3 d 5 ) B. ( 1 s 2 2 p 6 3 s 3 3 p 6 4 s 2 3 d 3 ) C. ( 1 s 2 2 p 6 3 s 2 3 p 6 4 s 1 3 d 5 ) D. ( 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 4 )
![36 A ground state atom of Mn2 has unpaired electrons and is A [36] A ground –state atom of Mn+2 has -----unpaired electrons and is -----. A.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-18.jpg)
[36] A ground –state atom of Mn+2 has -----unpaired electrons and is -----. A. 2, paramagnetic B. 4, diamagnetic C. 0, diamagnetic D. 5, paramagnetic [37] The orbital diagram for a ground state O 2 - atom is: A. ↓↑ ↓↑ ↓ ↑ ↑ 1 s 2 s 2 p B. ↓↑ ↓↑ ↑ ↑ 1 s 2 s 2 p C. ↓↑ ↓↑ ↑ 1 s 2 s 2 p D. ↓↑ ↓↑ ↓↑ 1 s 2 s 2 p
![38 Which two electron configurations represent elements that would have similar chemical properties 1 [38] Which two electron configurations represent elements that would have similar chemical properties? 1.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-19.jpg)
[38] Which two electron configurations represent elements that would have similar chemical properties? 1. 1 s 22 p 4 2. 1 s 22 p 5 3. [Ar]4 s 23 d 104 p 3 4. [Ar]4 s 23 d 104 p 4 A. (2) and (4) B. (1) and (2) C. (2) and (3) D. (1) and (4) [39] What is the electron configuration of the Mo 42? A. ( 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 104 p 65 s 14 d 9 ) B. ( 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 104 p 6 5 s 1 4 d 5) C. ( 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 104 p 64 d 9 5 s 2) D. ( 1 s 2 2 p 6 3 s 2 3 p 6 4 s 2 3 d 104 p 6 5 s 2 4 d 4
![40 Which of the orbital diagrams is not follow the pauli exclusion principle [40] Which of the orbital diagrams is not follow the pauli exclusion principle? ↑](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-20.jpg)
[40] Which of the orbital diagrams is not follow the pauli exclusion principle? ↑ ↑ ↑↑ ↑ ↑ ↓ ↑↓ ↑ ↓↓ ↑↓ ↑↓ ↑ ↑ ↑↓ A. (1) and(6) B. (2) and(5) C. (1) , (2)and(6) D. (3) , (4)and(5) [41] Which of the orbital diagrams is not follow Hand's rule? ↑ ↑ ↑↑ ↑ ↓ ↓ ↑↓ A. (1) and(4) B. (1) and(5) C. (2) and(3) D. (2) , (4)and(6) ↑↓ ↑↓ ↑ ↓↓ ↑↓ ↑ ↑
![42Choose the incorrect quantum numbers for electron in an atom A 3 [42]Choose the incorrect quantum numbers for electron in an atom? A. (3 ,](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-21.jpg)
[42]Choose the incorrect quantum numbers for electron in an atom? A. (3 , 0 , +1/2) B. (3 , 5 , 0 , +1/2) C. (1 , 0 , +1/2) D. (4 , 3 , -2 , -1/2) [43] The number of orbital in a p subshell is? A. 1 B. 7 C. 5 D. 3 [44] The maximum number of electrons that can occupy an energy level described by the principal quantum number , n, is? A. n+1 B. 2 n C. n 2 D. 2 n 2
![45 The electron configuration of a neutral atom isNe 3 s 23 p 3 [45] The electron configuration of a neutral atom is[Ne] 3 s 23 p 3.](https://slidetodoc.com/presentation_image_h/82ed1d9f49899ec3f58648da61e27878/image-22.jpg)
[45] The electron configuration of a neutral atom is[Ne] 3 s 23 p 3. The four quantum numbers of the last electron are: A. (3 , 1 , +1/2 ) B. (2 , 0 , +1/2 ) C. (2 , 0 , -1 , +1/2 ) D. (3 , 2 , -1 , +1/2 ) 46 What is the energy (in joules) a photon must have in order to excite an electron from E 2 to E 3? A) 5 × 10– 10 J B) 10 × 10– 19 J C) 5 × 10– 19 J D) 15 × 10– 19 J 47. The atomic number of an element is 73. Is this element diamagnetic or paramagnetic? (A) Diamagnetic (B) Paramagnetic

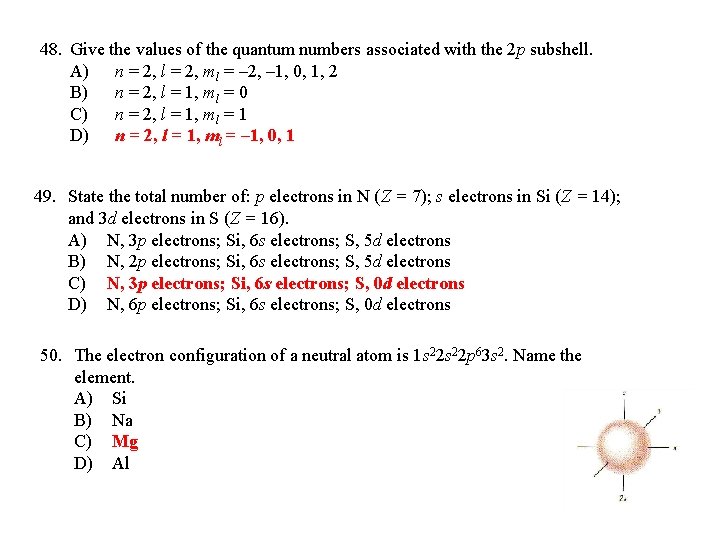
48. Give the values of the quantum numbers associated with the 2 p subshell. A) n = 2, l = 2, ml = – 2, – 1, 0, 1, 2 B) n = 2, l = 1, ml = 0 C) n = 2, l = 1, ml = 1 D) n = 2, l = 1, ml = – 1, 0, 1 49. State the total number of: p electrons in N (Z = 7); s electrons in Si (Z = 14); and 3 d electrons in S (Z = 16). A) N, 3 p electrons; Si, 6 s electrons; S, 5 d electrons B) N, 2 p electrons; Si, 6 s electrons; S, 5 d electrons C) N, 3 p electrons; Si, 6 s electrons; S, 0 d electrons D) N, 6 p electrons; Si, 6 s electrons; S, 0 d electrons 50. The electron configuration of a neutral atom is 1 s 22 p 63 s 2. Name the element. A) Si B) Na C) Mg D) Al

51. Which of the following species has the most unpaired electrons? S+, S, or S–? (A) S+ (B) S (C) S– (D) They all have the same number of unpaired electrons. 52. Use the Aufbau principle to obtain the ground-state electron configuration of selenium. A) Se: [Ar]4 s 23 d 104 p 3 B) Se: [Ar]4 s 23 d 104 p 4 C) Se: [Ar]4 s 23 d 104 p 5 D) Se: [Ar]4 s 23 d 104 p 6 54. How many orbitals are allowed in a subshell if the angular momentum quantum number for electrons in that subshell is 3? A. 1 B. 3 C. 5 D. 7 E. 9

55. How many electrons in a ground-state cadmium atom are in orbitals labeled by ml = -1? A. 2 B. 10 C. 12 D. 18 E. 36 56. A ground-state atom of iron has ___ unpaired electrons and is _____. A. 0, diamagnetic B. 6, diamagnetic C. 3, paramagnetic D. 5, paramagnetic E. 4, paramagnetic 57. Transition metal elements have atoms or ions with partially filled A. s subshells. B. p subshells. C. d subshells. D. f subshells. E. g subshells.
 Où se trouve le numéro d'affiliation mutuelle vignette ?
Où se trouve le numéro d'affiliation mutuelle vignette ? 110 000 110 111 000 111
110 000 110 111 000 111 Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Energy energy transfer and general energy analysis
Energy energy transfer and general energy analysis Active revision vs passive revision
Active revision vs passive revision How to find one mole of a compound
How to find one mole of a compound Ap chem spontaneity entropy and free energy
Ap chem spontaneity entropy and free energy General revision of assessments and property classification
General revision of assessments and property classification Tsbde rules and regulations
Tsbde rules and regulations Ib chemistry functional groups
Ib chemistry functional groups Inorganic chemistry vs organic chemistry
Inorganic chemistry vs organic chemistry General chemistry with qualitative analysis
General chemistry with qualitative analysis General chemistry thermochemistry
General chemistry thermochemistry General chemistry nomenclature
General chemistry nomenclature General chemistry 2
General chemistry 2 Organik kimya adlandırma öncelik sırası
Organik kimya adlandırma öncelik sırası General chemistry 11th edition
General chemistry 11th edition General chemistry 1 stoichiometry
General chemistry 1 stoichiometry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry General chemistry
General chemistry Chapter 7 energy conservation of energy
Chapter 7 energy conservation of energy Diferencia entre gran plano general y plano general
Diferencia entre gran plano general y plano general Where did general lee surrender to general grant?
Where did general lee surrender to general grant?