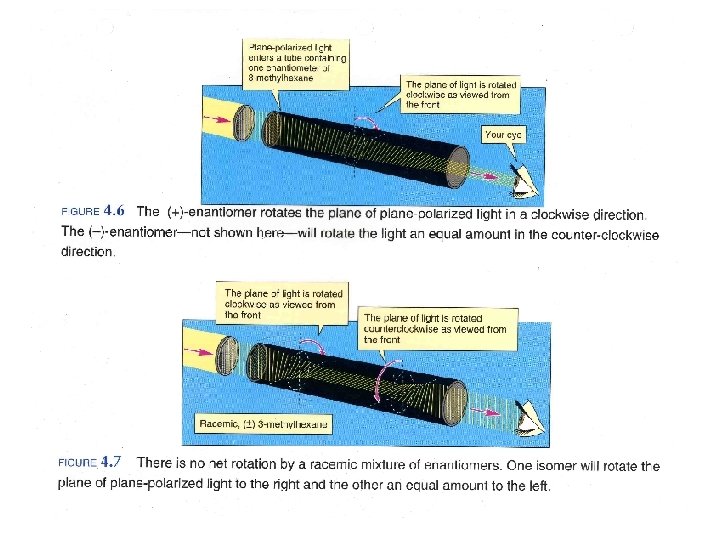
Enantiomers rotate plane polarized light the same magnitude





- Slides: 20




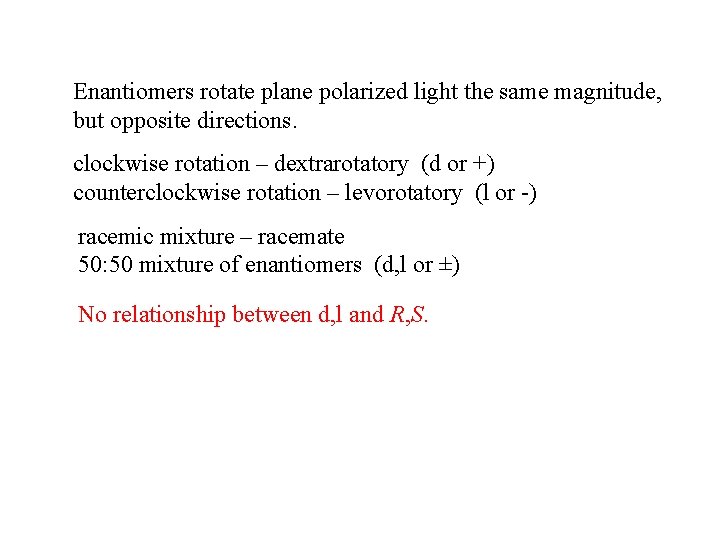
Enantiomers rotate plane polarized light the same magnitude, but opposite directions. clockwise rotation – dextrarotatory (d or +) counterclockwise rotation – levorotatory (l or -) racemic mixture – racemate 50: 50 mixture of enantiomers (d, l or ±) No relationship between d, l and R, S.


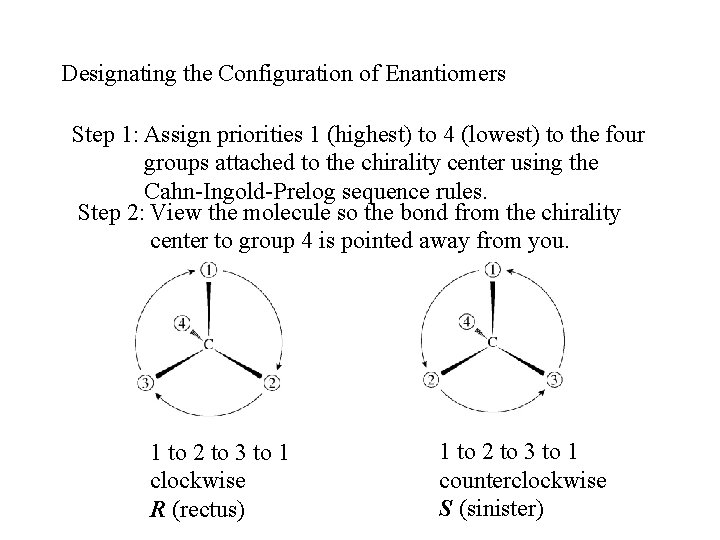
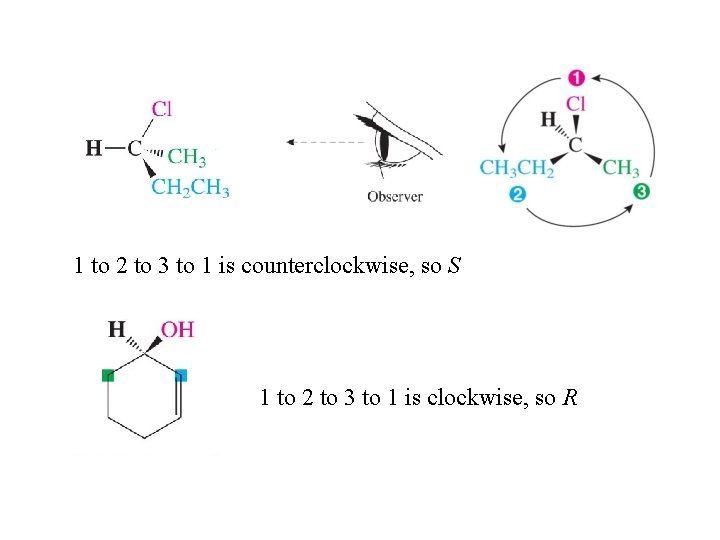
Designating the Configuration of Enantiomers Step 1: Assign priorities 1 (highest) to 4 (lowest) to the four groups attached to the chirality center using the Cahn-Ingold-Prelog sequence rules. Step 2: View the molecule so the bond from the chirality center to group 4 is pointed away from you. 1 to 2 to 3 to 1 clockwise R (rectus) 1 to 2 to 3 to 1 counterclockwise S (sinister)

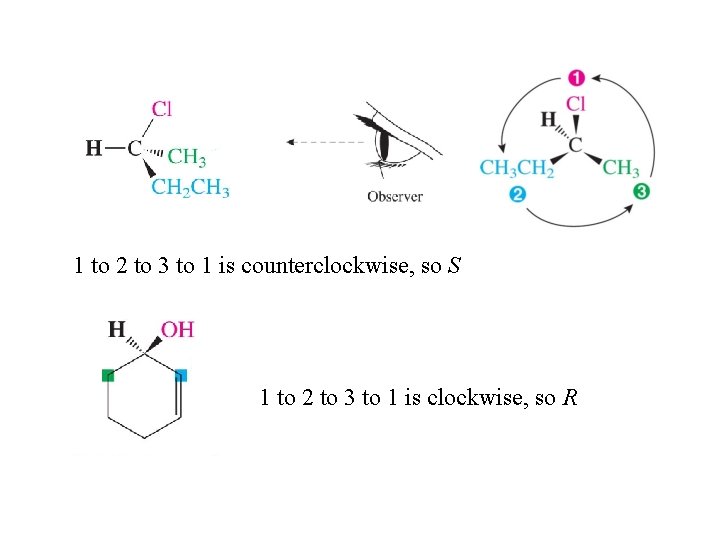
1 to 2 to 3 to 1 is counterclockwise, so S 1 to 2 to 3 to 1 is clockwise, so R

1 to 2 to 3 to 1 is counterclockwise, so S 1 to 2 to 3 to 1 is clockwise, so R

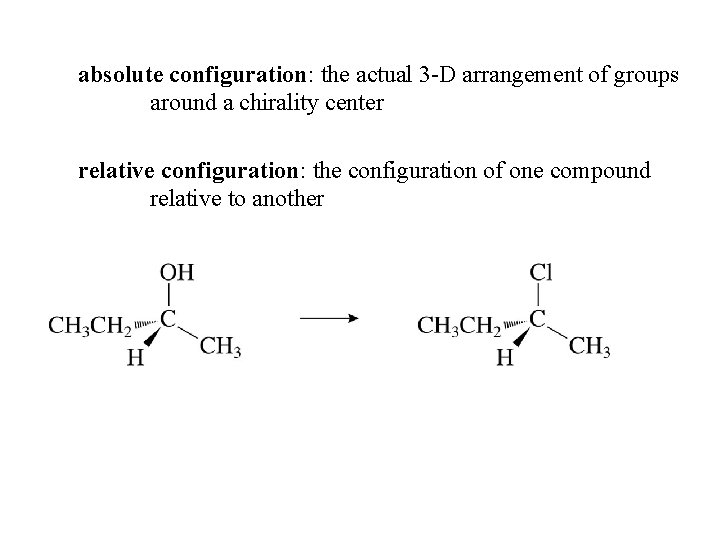
absolute configuration: the actual 3 -D arrangement of groups around a chirality center relative configuration: the configuration of one compound relative to another

absolute configuration: the actual 3 -D arrangement of groups around a chirality center relative configuration: the configuration of one compound relative to another

Properties of Enantiomers Enantiomeric molecules are only different in a chiral environment. They have identical mp, bp, heat of combustion, solubility in H 2 O They have different solubility in one enantiomer of a chiral solvent rate of reaction with one enantiomer of a chiral reagent

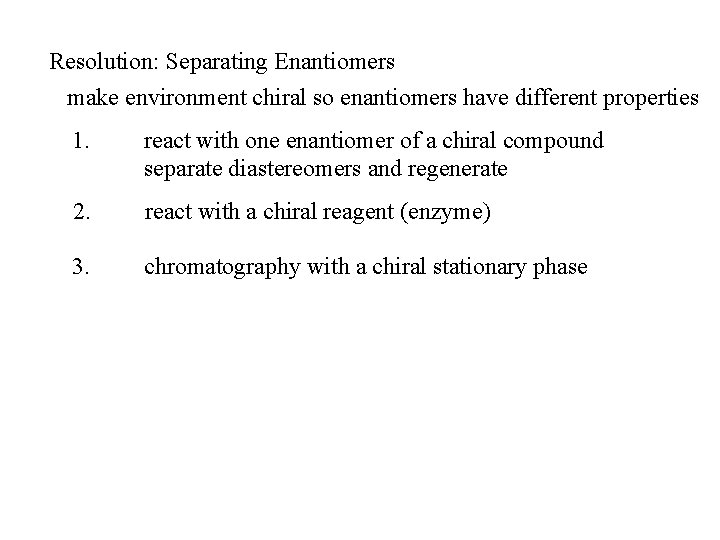
Resolution: Separating Enantiomers make environment chiral so enantiomers have different properties 1. react with one enantiomer of a chiral compound separate diastereomers and regenerate 2. react with a chiral reagent (enzyme) 3. chromatography with a chiral stationary phase

enantiomers to be separated one enantiomer of a chiral amine diastereomeric salts one diastereomer one pure enantiomer

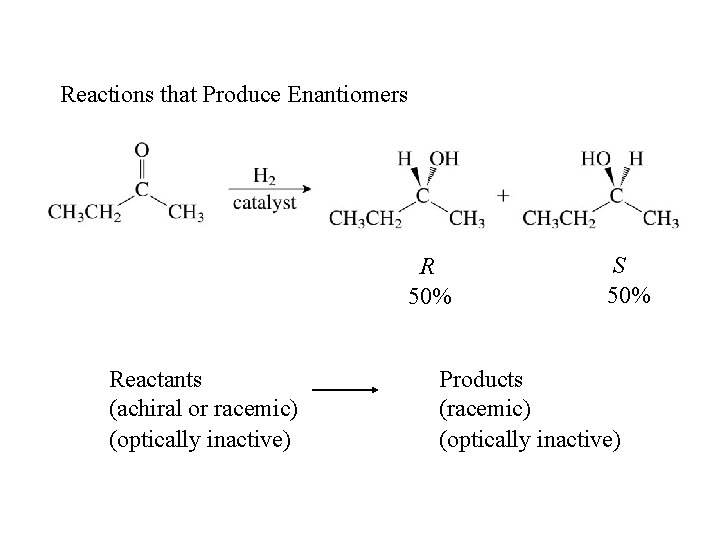
Reactions that Produce Enantiomers R 50% Reactants (achiral or racemic) (optically inactive) S 50% Products (racemic) (optically inactive)

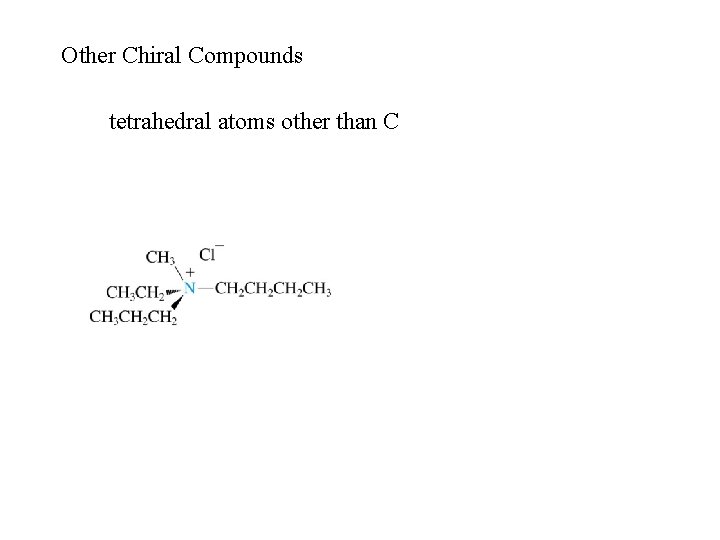
Other Chiral Compounds tetrahedral atoms other than C

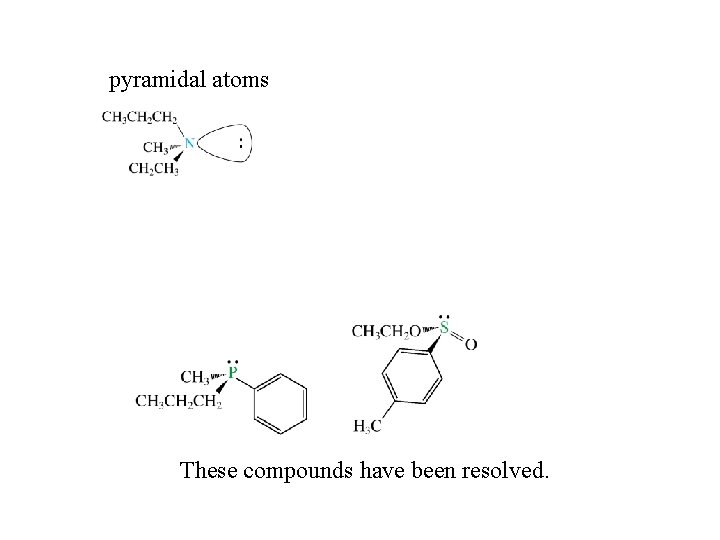
pyramidal atoms These compounds have been resolved.

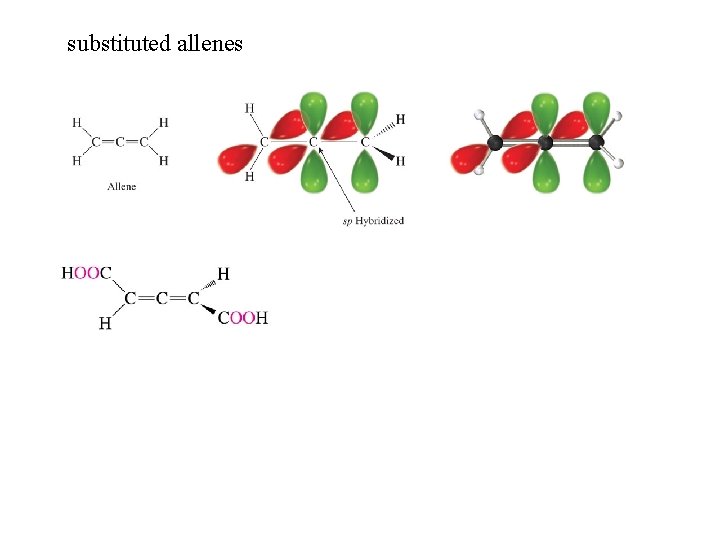
substituted allenes

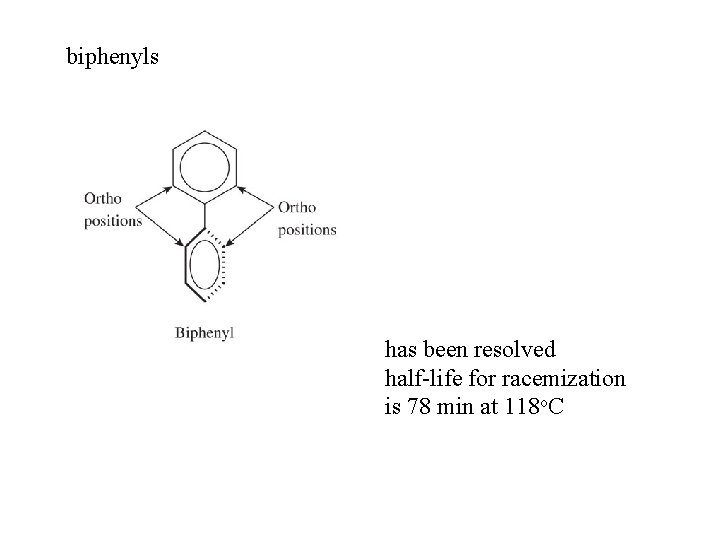
biphenyls has been resolved half-life for racemization is 78 min at 118 o. C

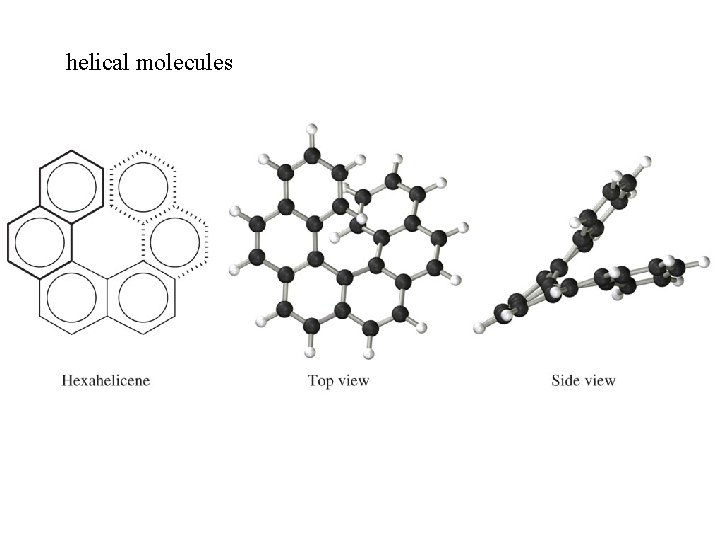
helical molecules
