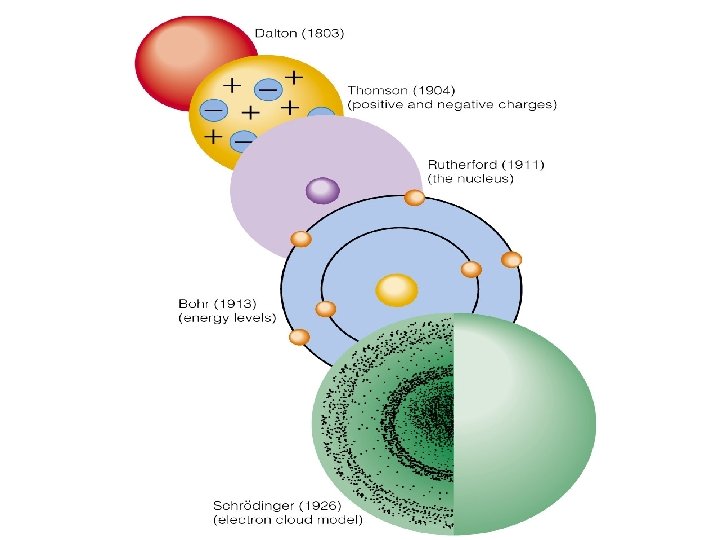
TOPIC Electrons in Atoms GroundExcited States Do Now


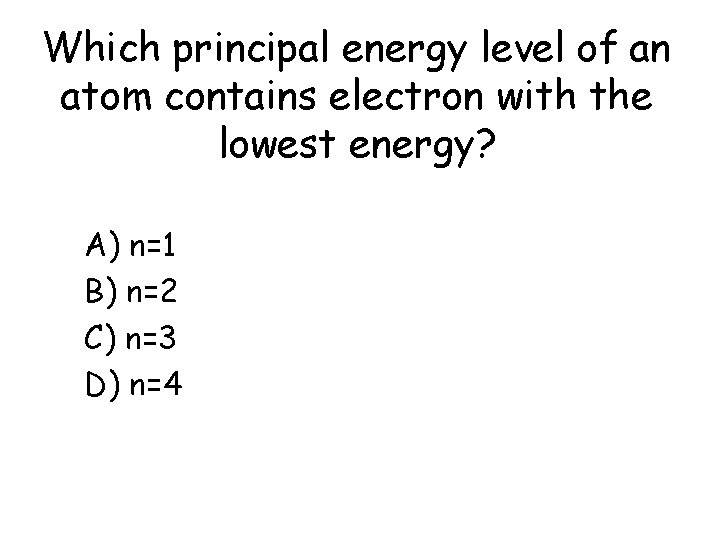
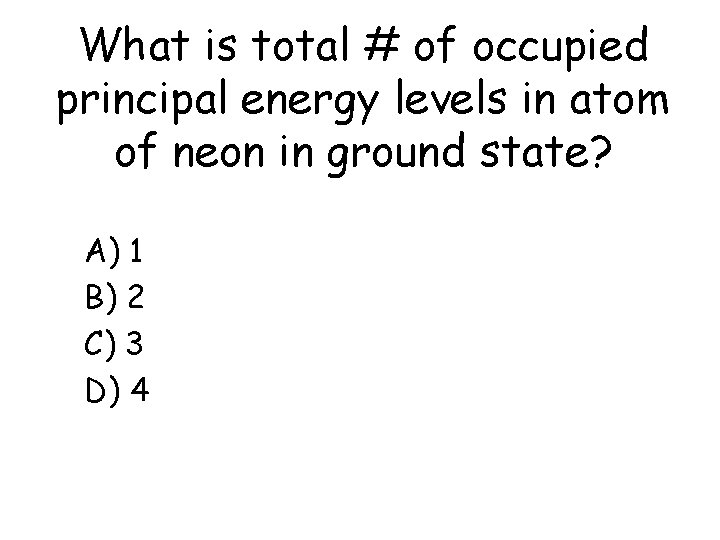

- Slides: 20

TOPIC: Electrons in Atoms Ground/Excited States Do Now: Draw Bohr’s Model for Argon


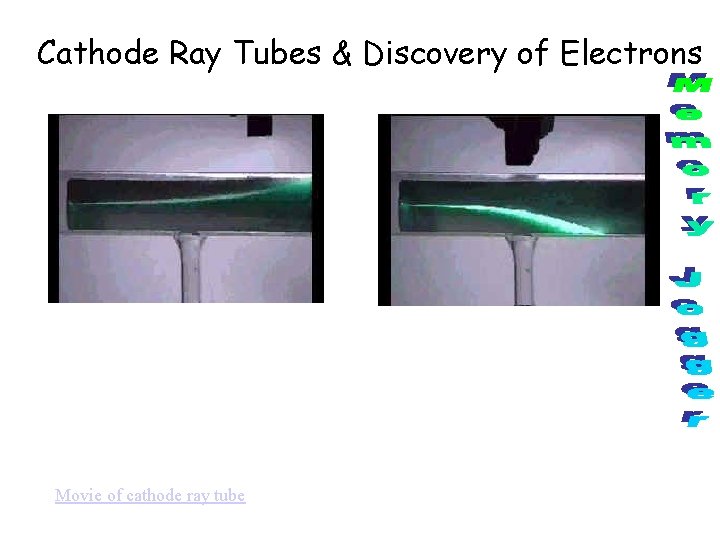
Cathode Ray Tubes & Discovery of Electrons Movie of cathode ray tube

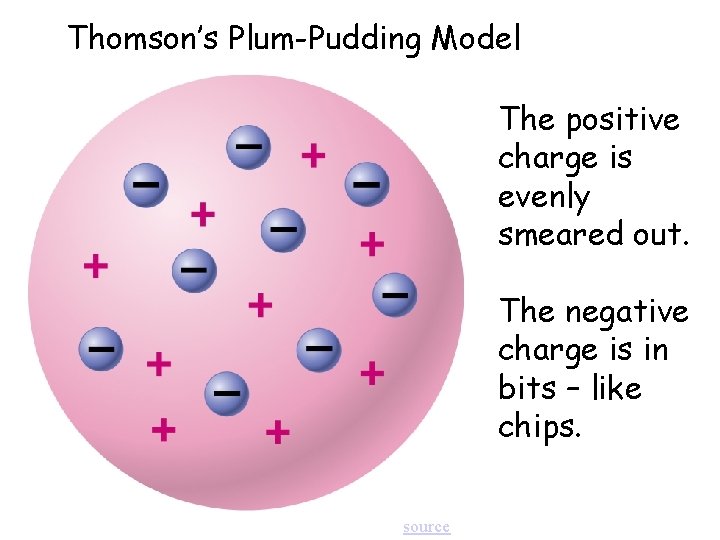
Thomson’s Plum-Pudding Model The positive charge is evenly smeared out. The negative charge is in bits – like chips. source

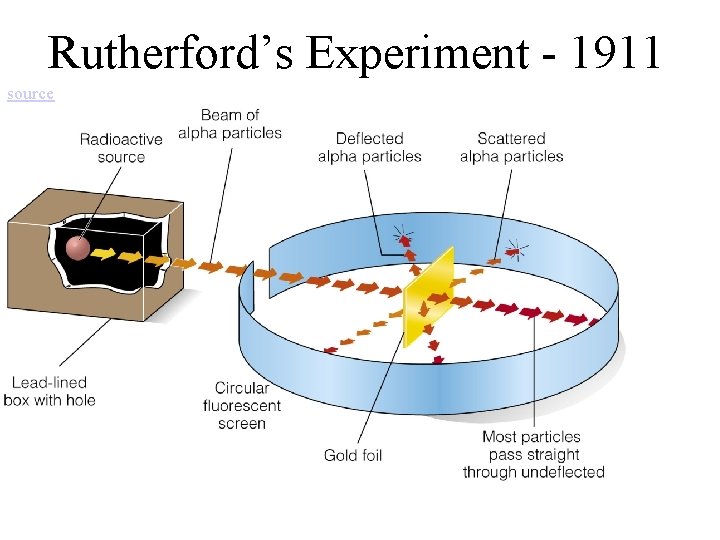
Rutherford’s Experiment - 1911 source

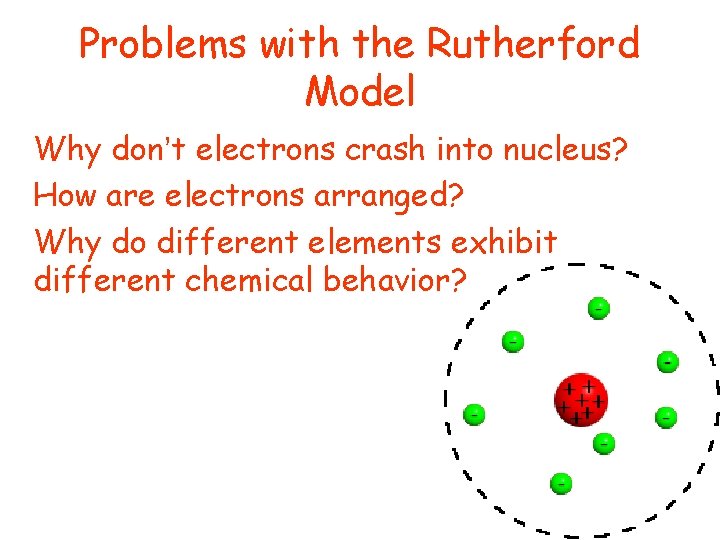
Problems with the Rutherford Model • Why don’t electrons crash into nucleus? • How are electrons arranged? • Why do different elements exhibit different chemical behavior?

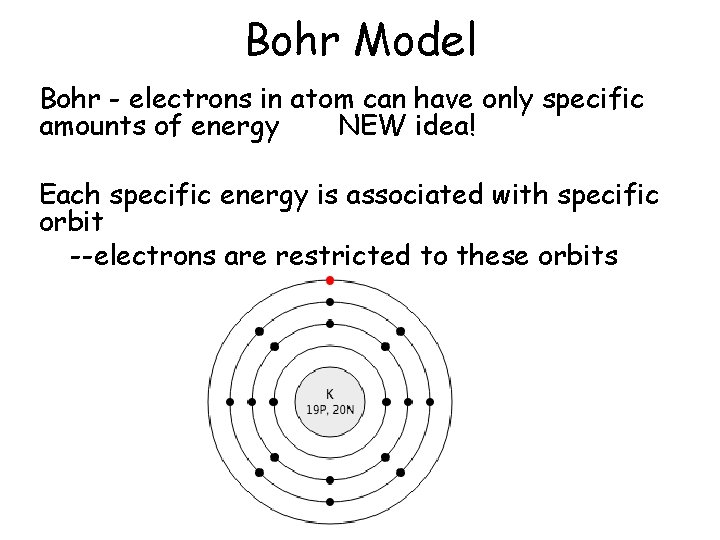
Bohr Model • Bohr - electrons in atom can have only specific amounts of energy NEW idea! • Each specific energy is associated with specific orbit – --electrons are restricted to these orbits

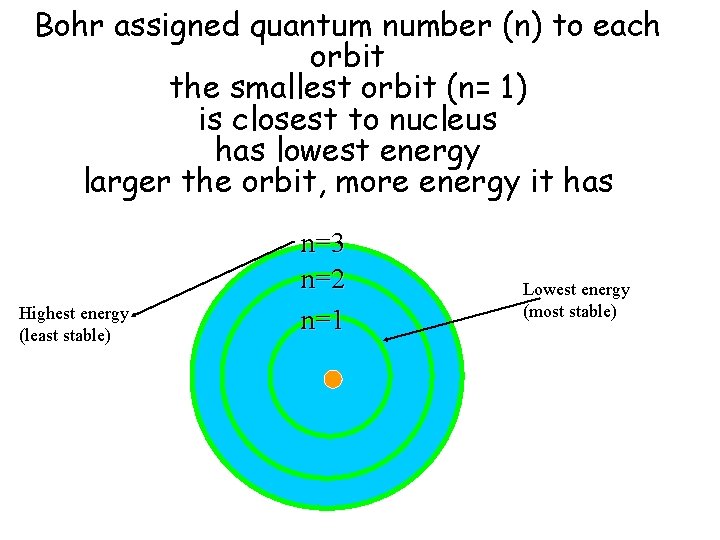
Bohr assigned quantum number (n) to each orbit the smallest orbit (n= 1) is closest to nucleus has lowest energy larger the orbit, more energy it has Highest energy (least stable) n=3 n=2 n=1 Lowest energy (most stable)

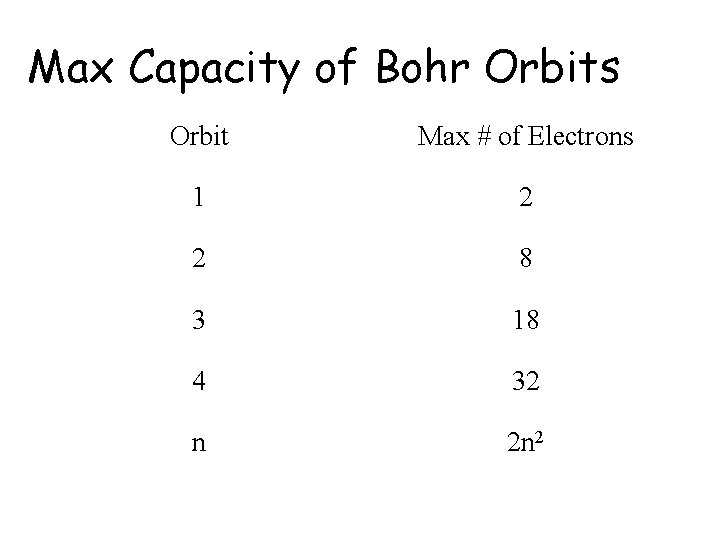
Max Capacity of Bohr Orbits Orbit Max # of Electrons 1 2 2 8 3 18 4 32 n 2 n 2

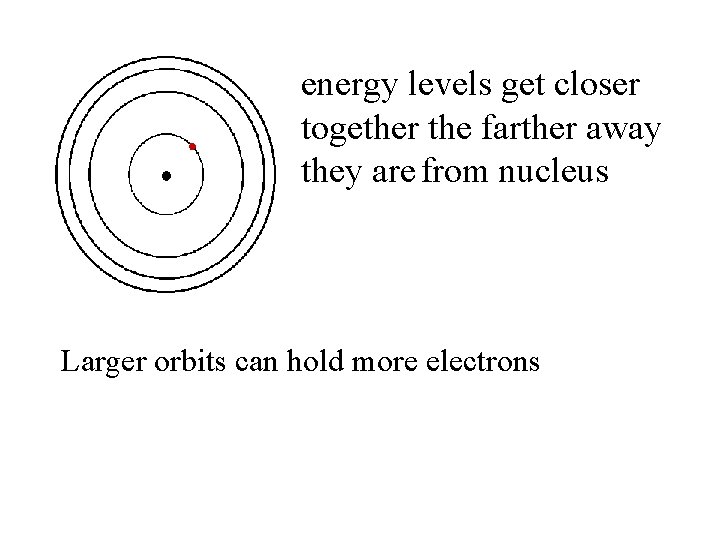
energy levels get closer together the farther away they are from nucleus Larger orbits can hold more electrons

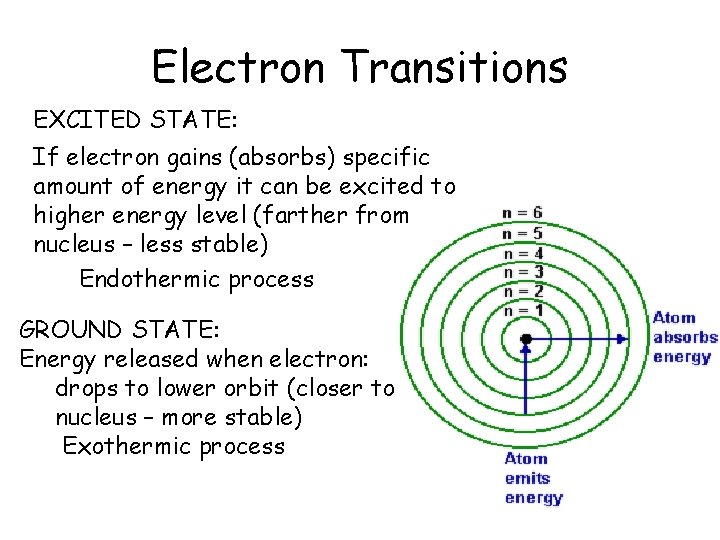
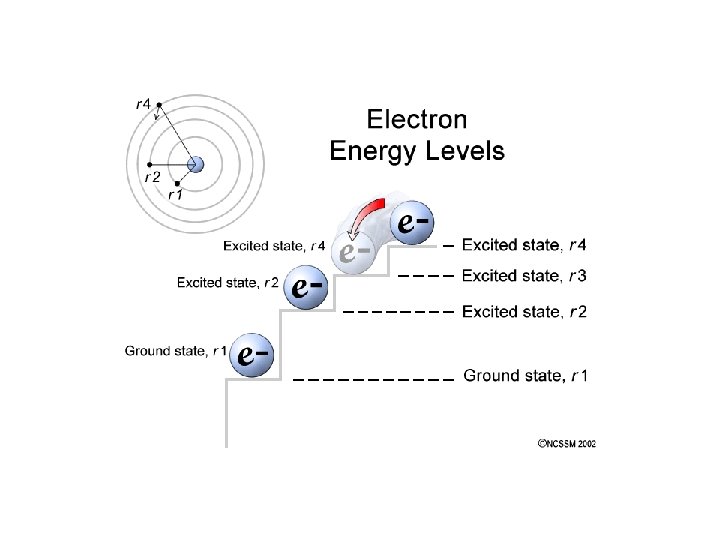
Electron Transitions • EXCITED STATE: • If electron gains (absorbs) specific amount of energy it can be excited to higher energy level (farther from nucleus – less stable) Endothermic process GROUND STATE: Energy released when electron: drops to lower orbit (closer to nucleus – more stable) Exothermic process

Ground State vs. Excited State • Ground state: – Lowest energy state of atom – electrons in lowest possible energy levels • Configurations in Reference Tables are ground state • Excited state: – Many possible excited states for each atom – One or more electrons excited to higher energy level


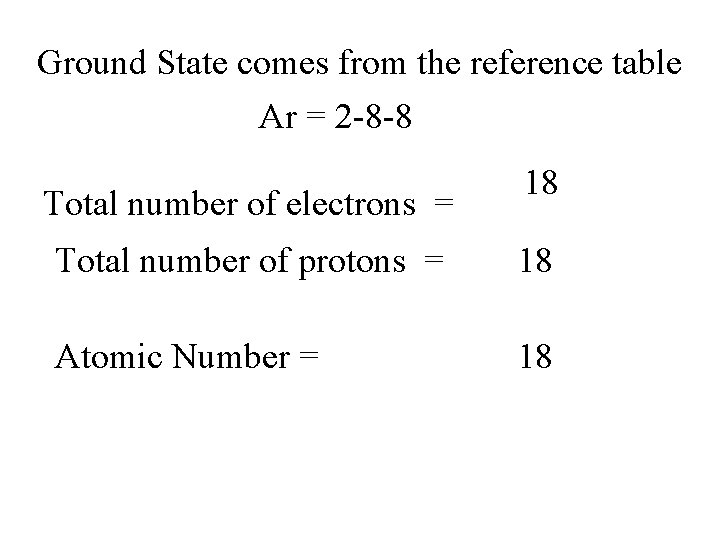
Ground State comes from the reference table Ar = 2 -8 -8 Total number of electrons = 18 Total number of protons = 18 Atomic Number = 18

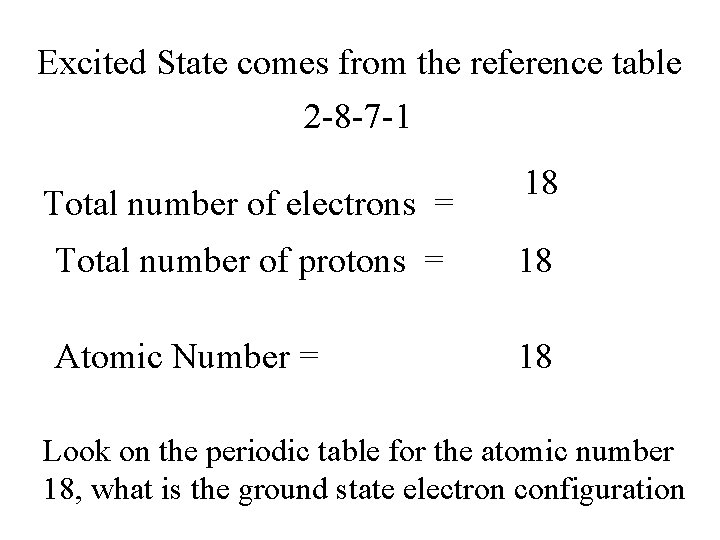
Excited State comes from the reference table 2 -8 -7 -1 Total number of electrons = 18 Total number of protons = 18 Atomic Number = 18 Look on the periodic table for the atomic number 18, what is the ground state electron configuration
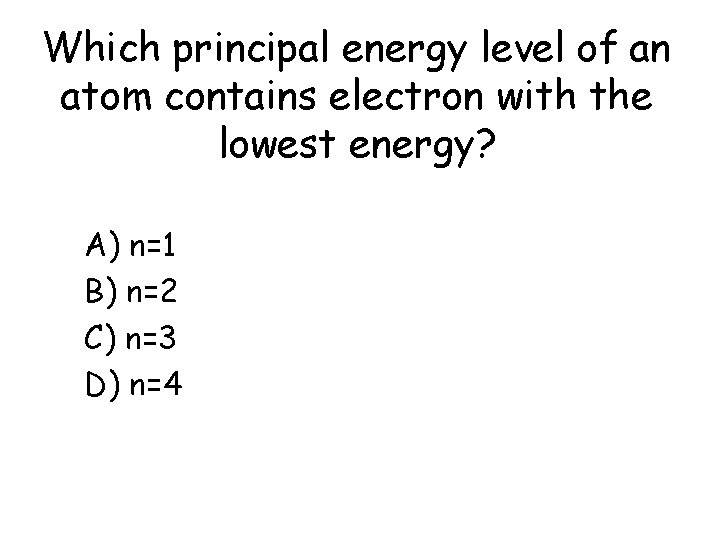
Which principal energy level of an atom contains electron with the lowest energy? a) b) c) d) A) n=1 B) n=2 C) n=3 D) n=4
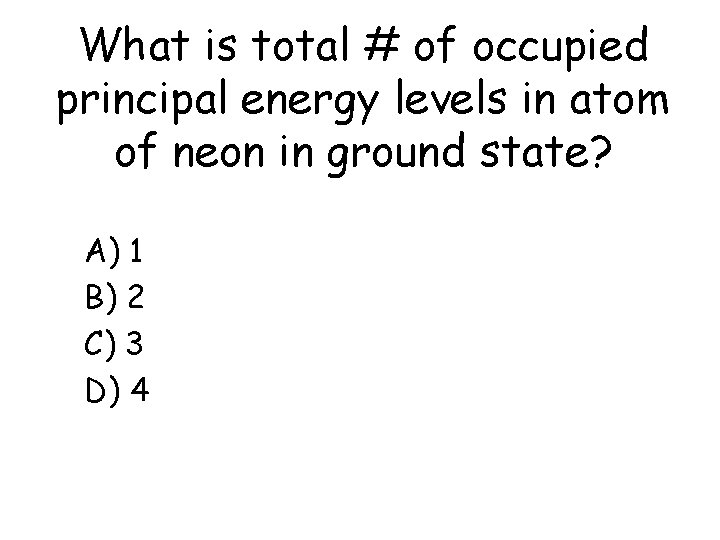
What is total # of occupied principal energy levels in atom of neon in ground state? a) b) c) d) A) 1 B) 2 C) 3 D) 4

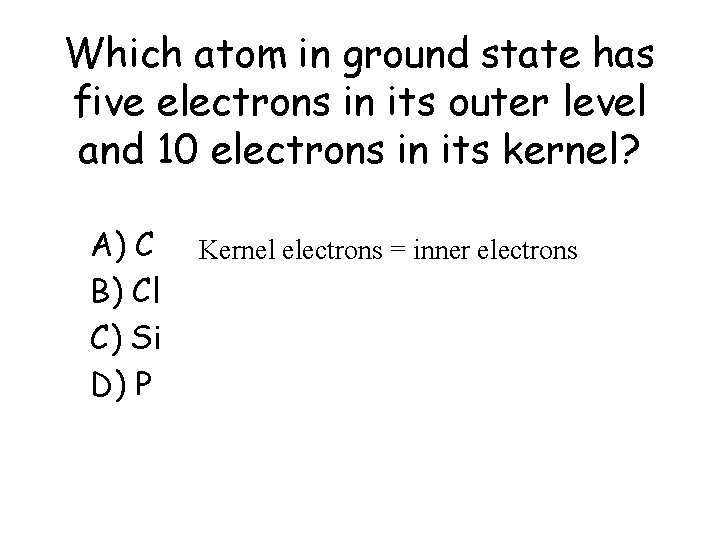
Which atom in ground state has five electrons in its outer level and 10 electrons in its kernel? a) b) c) d) A) C B) Cl C) Si D) P Kernel electrons = inner electrons

Which electron configuration represents atom in excited state? A) 2 -8 -2 a) B) 2 -8 -1 b) C) 2 -8 c) D) 2 -7 -1

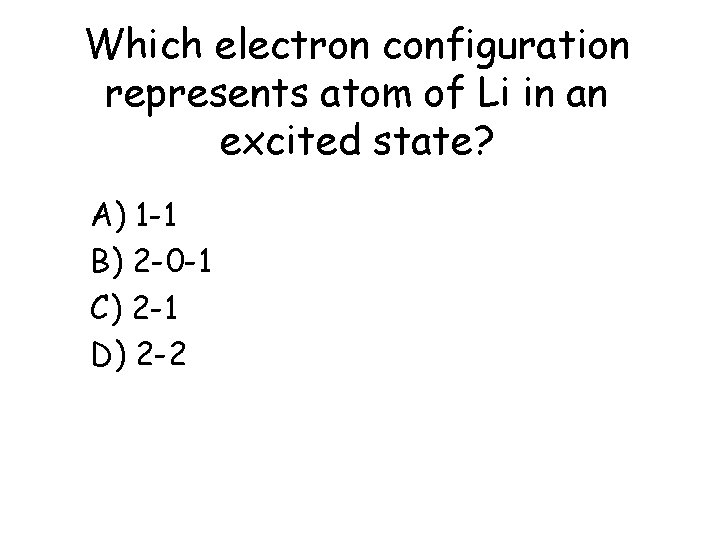
Which electron configuration represents atom of Li in an excited state? a) b) c) d) A) 1 -1 B) 2 -0 -1 C) 2 -1 D) 2 -2