Periodic Trends Types of Periodic Trends We are











- Slides: 21

Periodic Trends

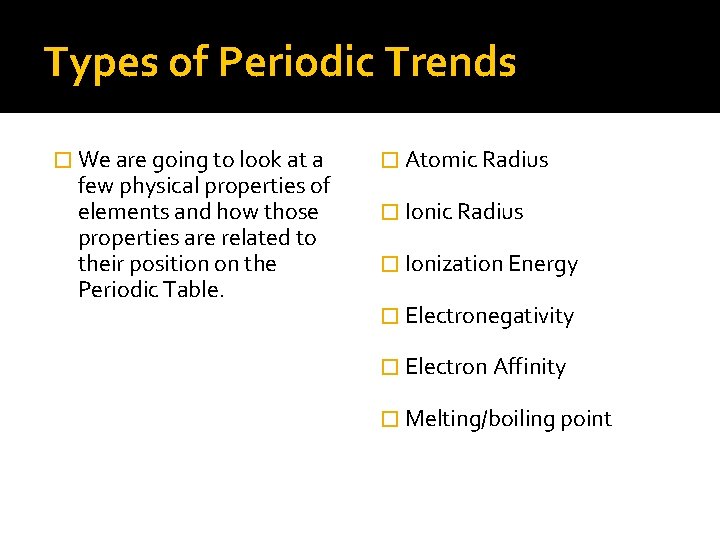
Types of Periodic Trends � We are going to look at a few physical properties of elements and how those properties are related to their position on the Periodic Table. � Atomic Radius � Ionization Energy � Electronegativity � Electron Affinity � Melting/boiling point

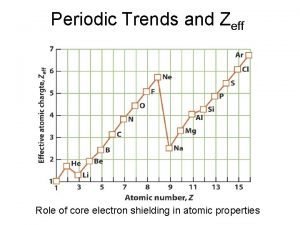
The Shielding Effect �The shielding effect describes the decrease in attraction between an electron and the nucleus in any atom with more than one electron shell. �The inner electrons act as a shield between the nucleus and the outer electrons so there is not as strong attraction.

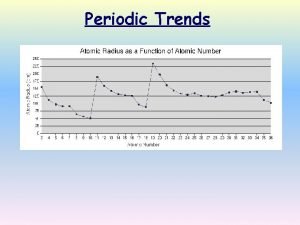
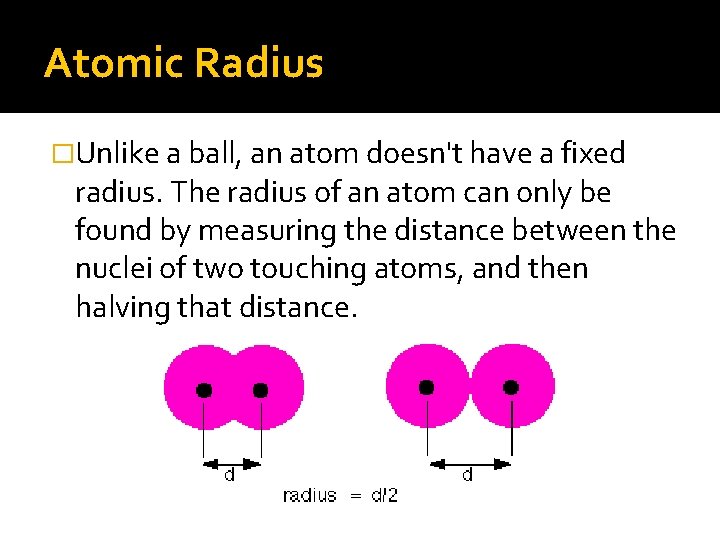
Atomic Radius �Unlike a ball, an atom doesn't have a fixed radius. The radius of an atom can only be found by measuring the distance between the nuclei of two touching atoms, and then halving that distance.

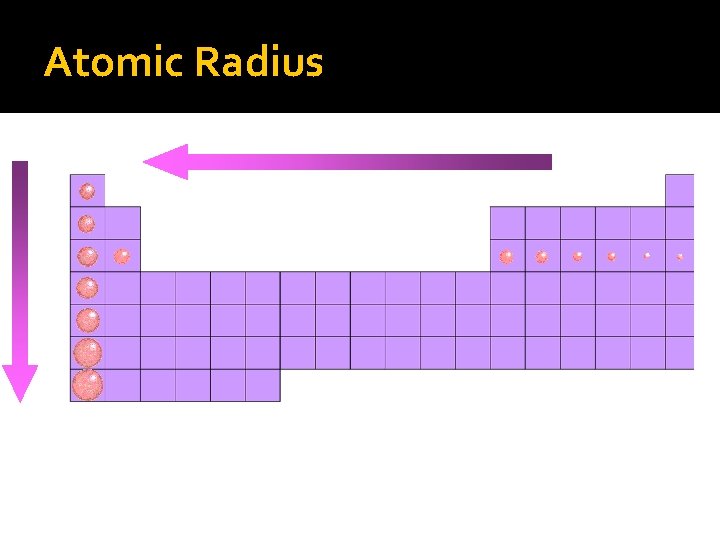
Atomic radii estimated size of the atom � Atomic size increases as you move down a group b/c e- are added to higher principal energy levels. SHEILDING EFFECT. � Atomic size decreases as you move from left to right across a period b/c e- are added to the same energy level. � The increasing force in the nucleus pulls the outermost e- closer to the center

Atomic Radius


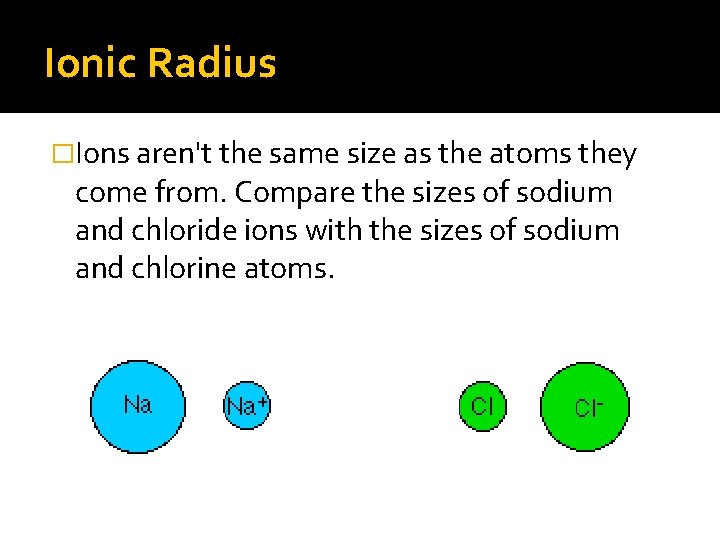
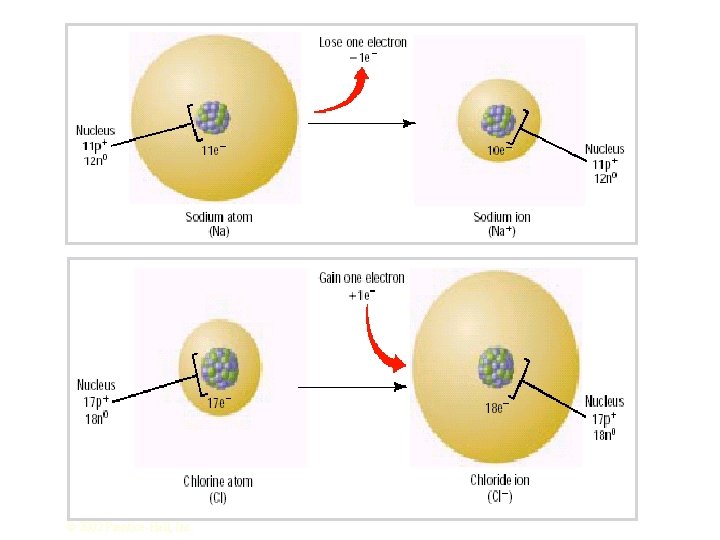
Ionic Radius �Ions aren't the same size as the atoms they come from. Compare the sizes of sodium and chloride ions with the sizes of sodium and chlorine atoms.

Positive ions �Positive ions are smaller than the atoms they come from. Sodium is 2, 8, 1; Na+ is 2, 8. You've lost a whole layer of electrons, and the remaining 10 electrons are being pulled in by the full force of 11 protons.

Negative ions �Negative ions are bigger than the atoms they come from. Chlorine is 2, 8, 7; Cl- is 2, 8, 8. Although the electrons are still all in the 3 level, the extra repulsion produced by the incoming electron causes the atom to expand. There are still only 17 protons, but they are now having to hold 18 electrons.

© 2002 Prentice-Hall, Inc.

Ionization Energy �Energy needed to remove an e- from an atom (1 st ionization energy); atom is now a + ion �Indicates how strongly an atom holds onto its electrons � 2 nd ionization energy- energy needed to remove a second e- (this takes more energy)

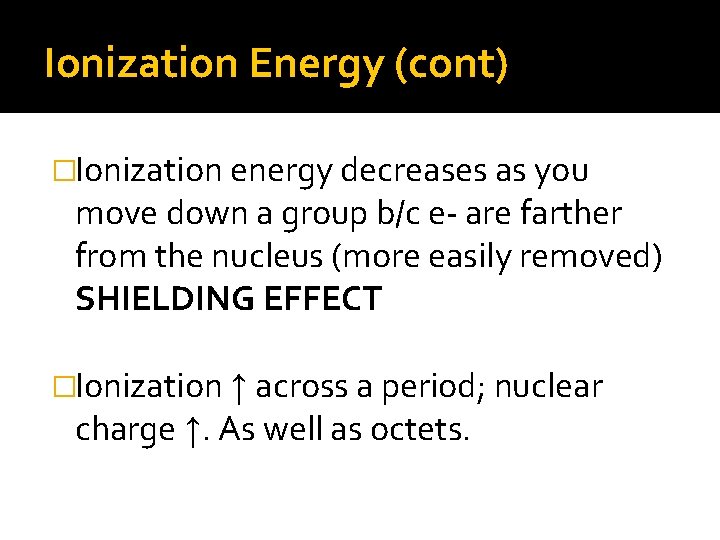
Ionization Energy (cont) �Ionization energy decreases as you move down a group b/c e- are farther from the nucleus (more easily removed) SHIELDING EFFECT �Ionization ↑ across a period; nuclear charge ↑. As well as octets.


Electronegativity �tendency for an atom to attract a pair of electrons to itself. �↑ across a period; ↓ as you move down a group. �Metals have low electronegativities. �Nonmetals have high electronegativities. �Trends in transition metals are not regular.


Electronegativity �Why do we see these trends? �It increases up a group because of the sheilding effect. �It increases across a period due to the number of valence electrons in the atom. The closer you get to having 8 the more you want an electron.

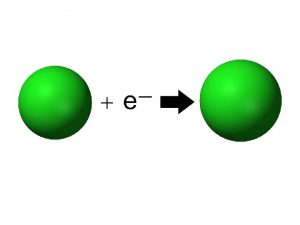
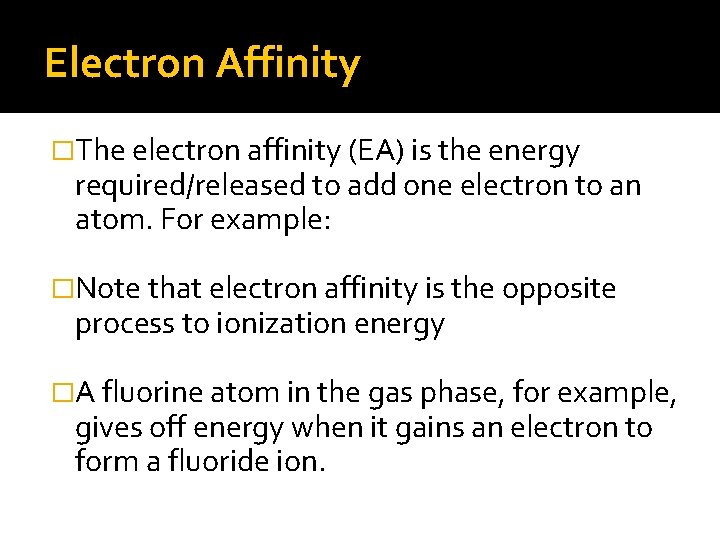
Electron Affinity �The electron affinity (EA) is the energy required/released to add one electron to an atom. For example: �Note that electron affinity is the opposite process to ionization energy �A fluorine atom in the gas phase, for example, gives off energy when it gains an electron to form a fluoride ion.


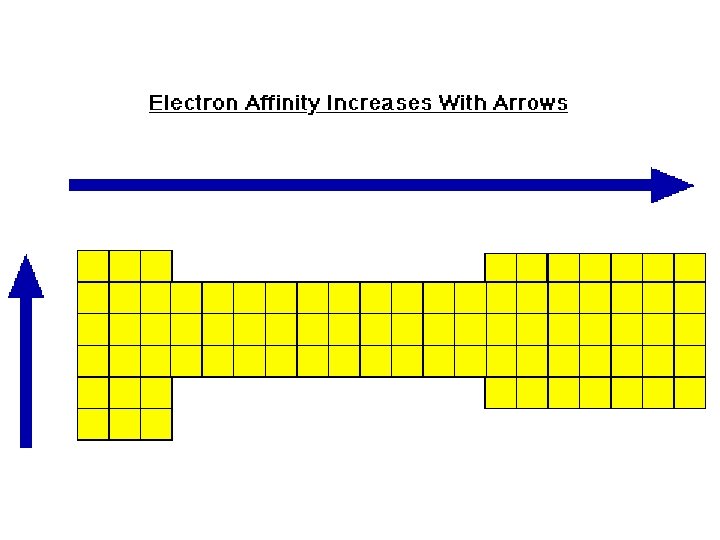
Electron Affinity � Electron affinities generally become smaller as we go down a column because the electron being added to the atom is placed in larger orbitals, where it spends less time near the nucleus of the atom and the shielding effect. � Affinities increase across a period because the atoms become more stable with the addition of an electron. The octet rule.

Melting/Boiling Point – Highest in the middle of a period.
 Antigentest åre
Antigentest åre Periodic tends
Periodic tends Alien periodic table periodic trends answers
Alien periodic table periodic trends answers As atomic size
As atomic size Trends on periodic table
Trends on periodic table Periodic table trends cheat sheet
Periodic table trends cheat sheet Oxygen periodic trends
Oxygen periodic trends Graphing periodic trends
Graphing periodic trends Oxidation trends periodic table
Oxidation trends periodic table Electronegativity meaning
Electronegativity meaning Zeff trend
Zeff trend Periodic trends activity worksheet
Periodic trends activity worksheet Periodic trends practice quiz
Periodic trends practice quiz Periodic trends in reactivity
Periodic trends in reactivity Periodic trends summary
Periodic trends summary Periodic trends practice problems
Periodic trends practice problems Coulomb's law periodic trends
Coulomb's law periodic trends Periodic table trends electron affinity
Periodic table trends electron affinity Increasing atomic size
Increasing atomic size Atomic radius trend
Atomic radius trend Definition of periodic trend
Definition of periodic trend Acidity trends periodic table
Acidity trends periodic table