Lesson 12 What is the difference between atomic

Lesson 12 What is the difference between atomic mass and atomic number?

Atoms of different elements have different numbers of protons and electrons. When scientists talk about different kinds of matter, they often refer to the matter by its atomic number.

The atomic number is the number of protons in the atom.
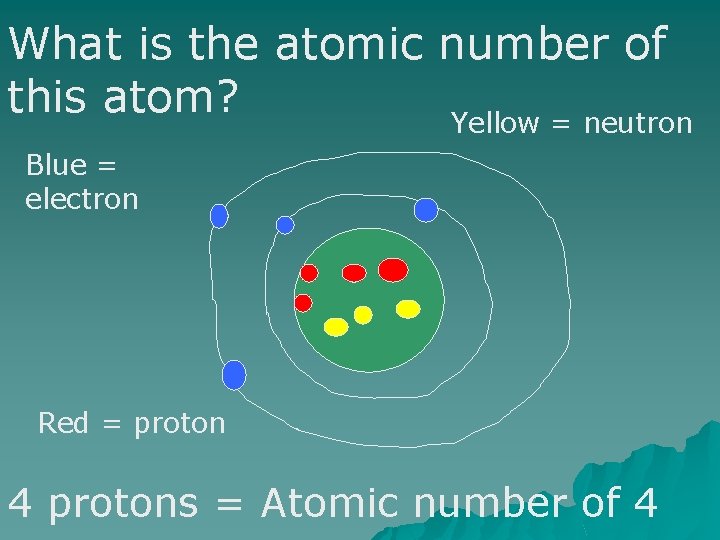
What is the atomic number of this atom? Yellow = neutron Blue = electron Red = proton 4 protons = Atomic number of 4

Scientists also describe atoms by their atomic mass. Scientists do not measure the mass of atoms in grams or ounces.

They use Atomic Mass Units or a. m. u. Each proton has a mass of 1 a. m. u. Each neutron has a mass of 1 a. m. u.

So………………. . The Atomic Mass of an atom is the number of protons plus the number of neutrons in the atom.
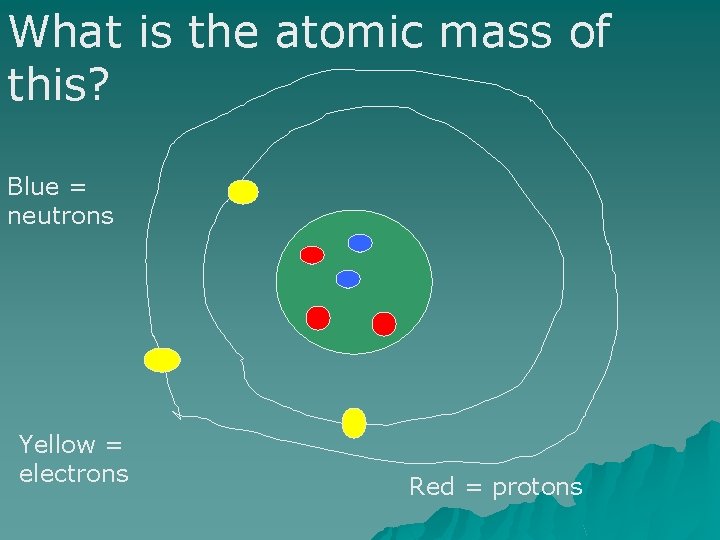
What is the atomic mass of this? Blue = neutrons Yellow = electrons Red = protons

Atomic mass = protons + neutrons. 3+2=5 Atomic mass = 5

Can you figure out why scientists don’t count electrons in the atomic mass? Think back to what you know about electrons. Anyone? ?

Electrons are very, very light. They have almost no mass. Therefore, scientists don’t count them in the atomic mass. Sometimes, two atoms of the same kind of matter do not have the same atomic mass. How in the world is that possible? ? ?
They have a different number of neutrons. Atoms of the same type of matter always have the same number of protons. When the number of neutrons is different, it makes the atomic mass different.

The atoms still have the same atomic number. (the number of protons is the same) Isotopes are atoms of the same kind of matter that have different number of neutrons.
- Slides: 13