Atomic Structure Basic Atomic Structure Basic Atomic Structure

Atomic Structure
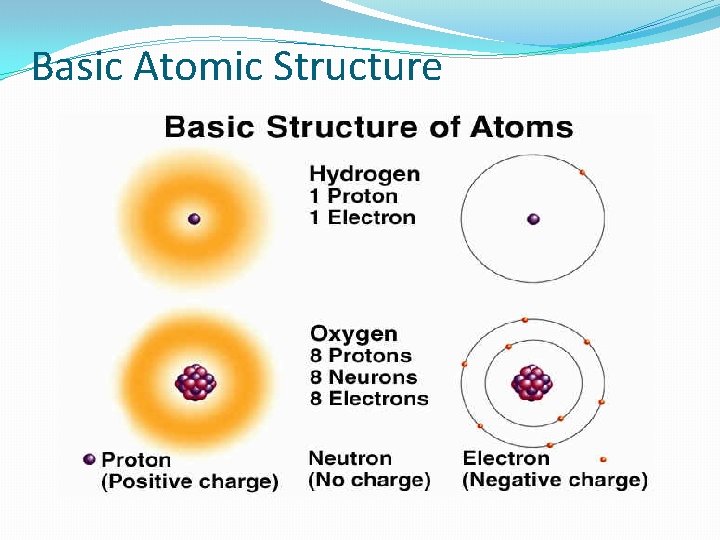
Basic Atomic Structure
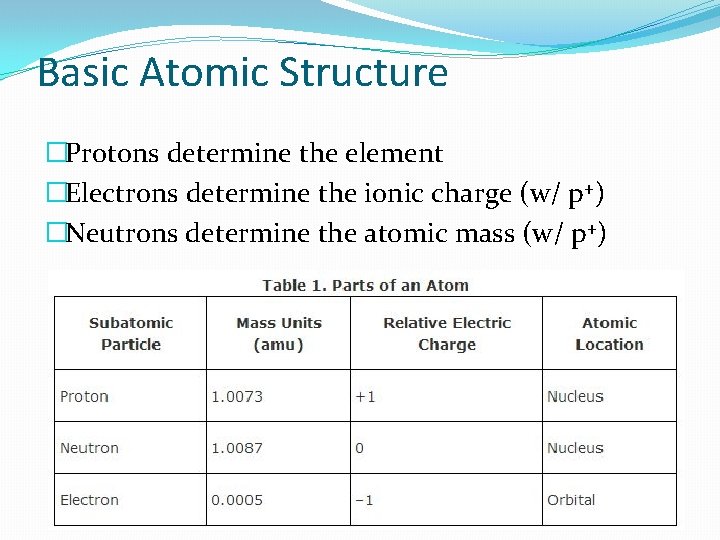
Basic Atomic Structure �Protons determine the element �Electrons determine the ionic charge (w/ p+) �Neutrons determine the atomic mass (w/ p+)
Atomic Number �The atomic number is the amount of protons for an elemental atom. �Atomic number or protons determine the type of element that is present. �In a neutral atom, the number of protons will equal the amount of electrons.
Atomic Mass �Mass number is the total number of protons and neutrons in an atom. �Mass Number = protons + neutrons �Mass number - protons = Number of neutrons �What is the mass of an element that has atom that has 12 protons and 14 neutrons? �How many neutrons does an element have if it has a mass of 47 and contains 22 protons?
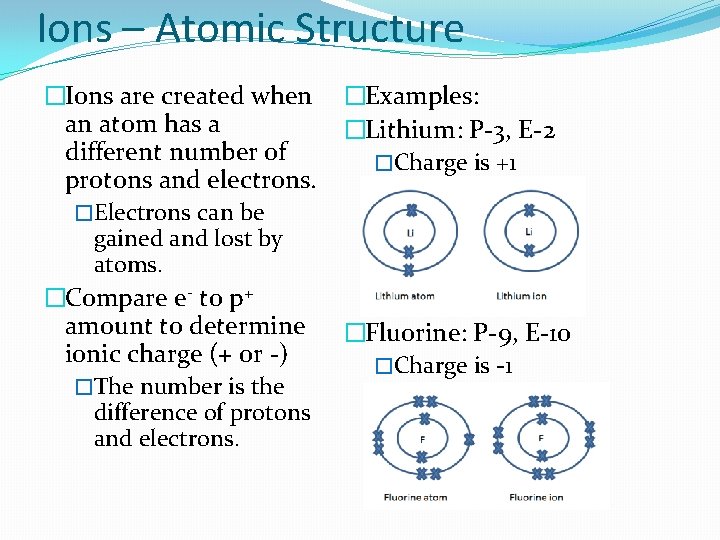
Ions – Atomic Structure �Ions are created when an atom has a different number of protons and electrons. �Electrons can be gained and lost by atoms. �Compare e- to p+ amount to determine ionic charge (+ or -) �The number is the difference of protons and electrons. �Examples: �Lithium: P-3, E-2 �Charge is +1 �Fluorine: P-9, E-10 �Charge is -1
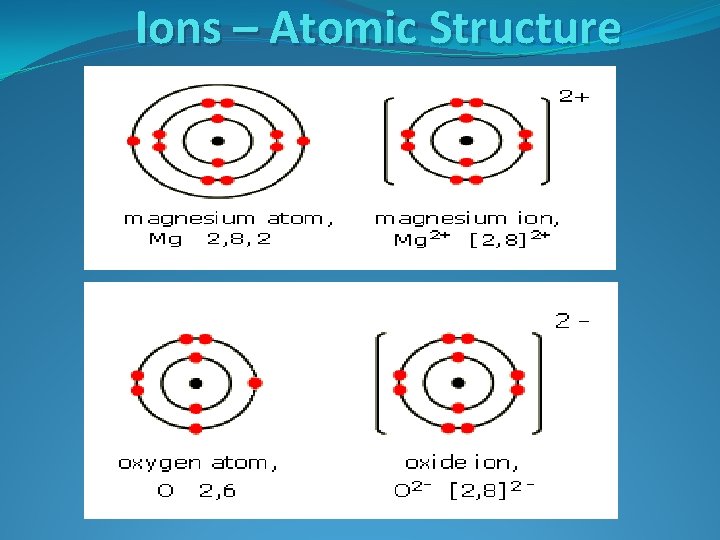
Ions – Atomic Structure
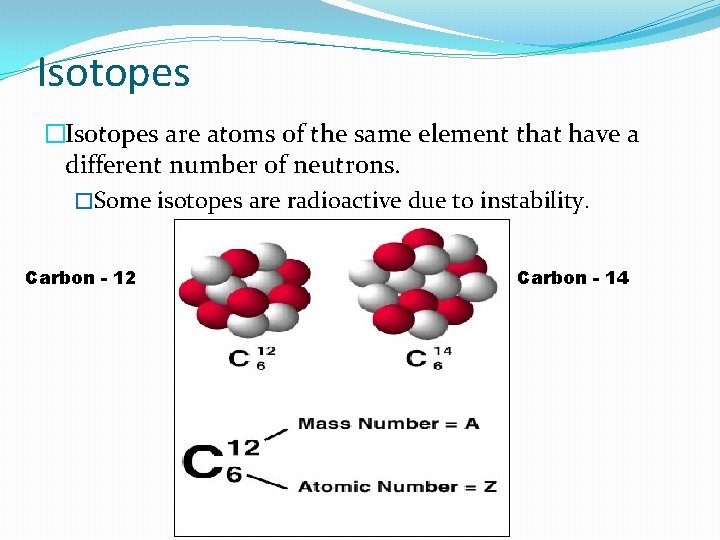
Isotopes �Isotopes are atoms of the same element that have a different number of neutrons. �Some isotopes are radioactive due to instability. Carbon - 12 Carbon - 14
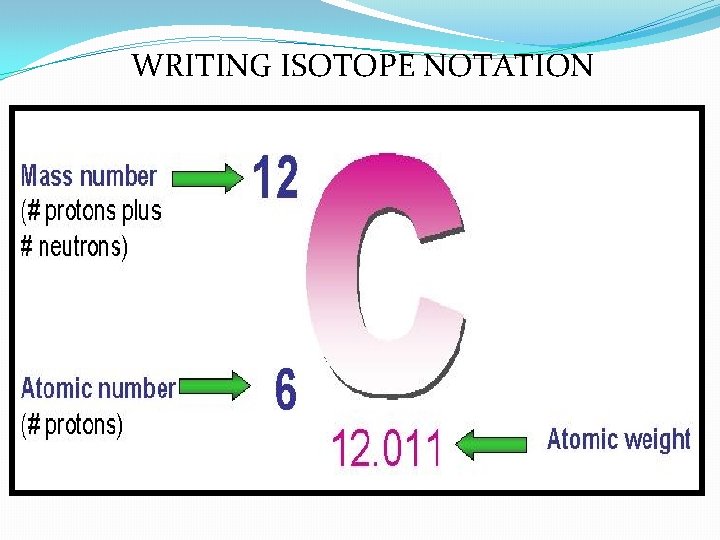
WRITING ISOTOPE NOTATION
�Practice Problems: �How many protons, neutrons, and electrons in Oxygen-16 �How many protons, neutrons, and electrons in Oxygen-17 �Write the name for the isotope 40 K. �Write the name for the isotope 235 U. �What is the charge of an atom that has 15 protons and 18 electrons? �What is the charge of an atom that has 31 protons and 28 electrons?
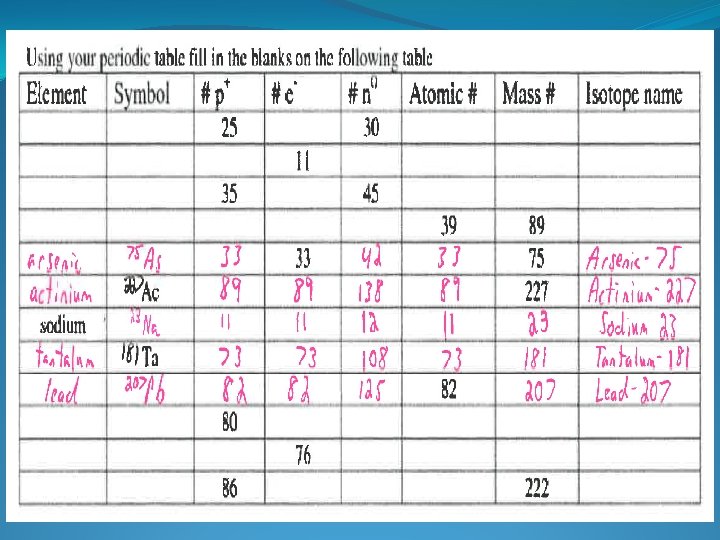
- Slides: 11