Atomic properties Atomic number atomic symbol mass number

Atomic properties Atomic number, atomic symbol, mass number, isotopes

HOW DO WE DESCRIBE AN ATOM We’ve already discussed what makes up an atom Proton Neutron Electron Now we need to find a way to describe the properties of an individual atom
DESCRIBING THE ATOM 1. 2. 3. Let’s take a look at the periodic tables around the room. On each periodic table are several things Symbol for the element Name of the element Numbers that describe the element

HOW DO WE NAME AN ELEMENT The first thing we will exam is the naming of elements Finding the names of elements is like doing a giant word search EXAMPLE: What is the name of the element with the symbol He?
ANSWER He is the symbol for the element: HELIUM

TRY THESE 1. 2. 3. 4. 5. 6. Br Li Zn B Ag K

ANSWERS 6. Br - Bromine Li - Lithium Zn - Zinc B - Boron Ag - Silver K – Potassium Let’s look at #5 and #6 1. 2. 3. 4. 5.

FIND THE SYMBOL FROM THE NAME 1. 2. 3. It’s usually easier to find the name of the element when you have the symbol. It’s harder to find the symbol when you have the name. Try these: Mercury Xenon Iron

ANSWERS 1. 2. 3. Mercury - Hg Xenon - Xe Iron – Fe REMINDER: For your periodic table You may use this on your quizzes You must fill in your own information

WHAT DEFINES AN ATOM One particle defines what an atom (or element is) This particle is the PROTON The number of protons in an atom tells us what element is.

ATOMIC NUMBER Atomic number: the number of protons in an atom Example: Find Hydrogen (H) on your periodic table The atomic number of hydrogen is 1. What is the atomic number for He?

ATOMIC NUMBER 1. 2. 3. The periodic table is arranged in order of increasing atomic number. Find the atomic number for these: Sulfur Uranium Gold

ATOMIC NUMBER 1. 2. 3. Sulfur - 16 Uranium - 92 Gold – 79 If you ever change the number of protons, you change the atom into a different element.

MASS NUMBER Mass number: the sum of an element’s protons and neutrons. Remember, electrons don’t have an atomic mass On your periodic table, this is found below the symbol of the element EXAMPLE: The mass number for H (hydrogen) is 1. 008

UNITS The unit for the mass unit is the atomic mass unit (amu) Therefore: Hydrogen has a mass number of 1. 008 amu

FIND THE MASS NUMBER 1. 2. 3. Find the mass number for the following: Cl Pd Sr

MASS NUMBER AND ISOTOPES Notice that not all the mass numbers are whole numbers A lot of them have decimals WHY?
ISOTOPES The reason is that not every atom in an element has the same number of neutrons ISOTOPE: atoms of the same element with different numbers of neutrons Isotopes of atoms is what is responsible for radiation The mass number is an average of all the different isotope masses
SYMBOLS How do we show many protons and neutrons are in a symbol. EXAMPLE: An element has 10 protons and 10 neutrons, how do we show it? First, what is the element?
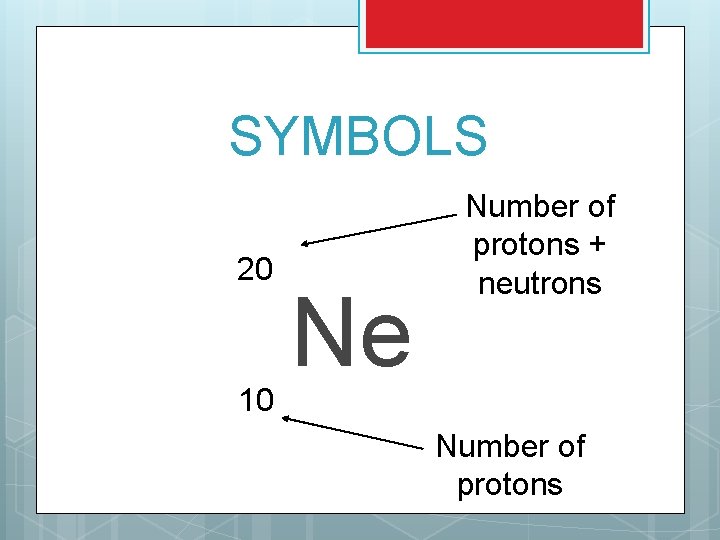
SYMBOLS 20 10 Ne Number of protons + neutrons Number of protons

WRITE THE SYMBOL FOR THESE 1. 2. 3. 35 protons and 45 neutrons 40 protons and 51 neutrons 78 protons and 117 neutrons

HOW MANY PROTONS AND NEUTRONS 40 Ar 18 56 Fe 26 238 U
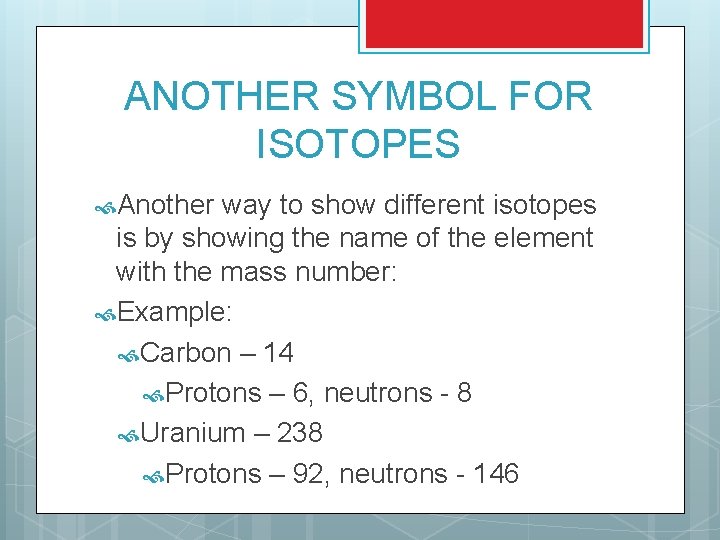
ANOTHER SYMBOL FOR ISOTOPES Another way to show different isotopes is by showing the name of the element with the mass number: Example: Carbon – 14 Protons – 6, neutrons - 8 Uranium – 238 Protons – 92, neutrons - 146

HOW MANY PROTONS AND NEUTRONS? Nitrogen – 14 Hydrogen – 3 Calcium - 41
- Slides: 24