Stoichiometry Modern Chemistry Holt Rinehart Winston 2009 Chapter




- Slides: 14

Stoichiometry Modern Chemistry, Holt, Rinehart, & Winston © 2009 Chapter 9, pp 298 -327.

Composition vs. Reaction Stoichiometry • Composition Stoichiometry • Deals with the mass relationships of elements in compounds • Law of definite proportions • Na. Cl always has 39. 34% sodium by mass & 60. 66% chlorine by mass. • Law of multiple proportions • For each 1. 0 g of carbon, there is 2. 66 g of oxygen in CO 2, but 1. 33 g of oxygen in CO. That gives a nice 2: 1 ratio for oxygen in these 2 compounds. • Reaction Stoichiometry • Involves mass relationships between reactants & products in reactions • This is accomplished by making ratios from the coefficients of the balanced equation. • Coefficients in balanced equations ALWAYS give the relative # of moles for each substance. 2 Al 2 O 3 4 Al + 3 O 2 This means there are 2 mol Al 2 O 3/ 4 mol Al; 2 mol Al 2 O 3/ 3 mol O 2; 4 mol Al/ 3 mol O 2 and all the reciprocals too. These are called ‘mole ratios’.

Mole Ratios & Molar Mass • Balanced equations give theoretical amounts for each substance. • Coefficients are interpreted as either # molecules or # relative moles, again in the best possible scenerio. • Real-world applications of chemical reactions seldom have exactly the # of moles as shown in a chemical reaction. • Real-world reactions are never 100% efficient (the theoretical yield). • We must measure in grams, liters, or cm 3 quantities used & produced. • Molar mass (grams/ mol) allow us to bridge theoretical with the actual.

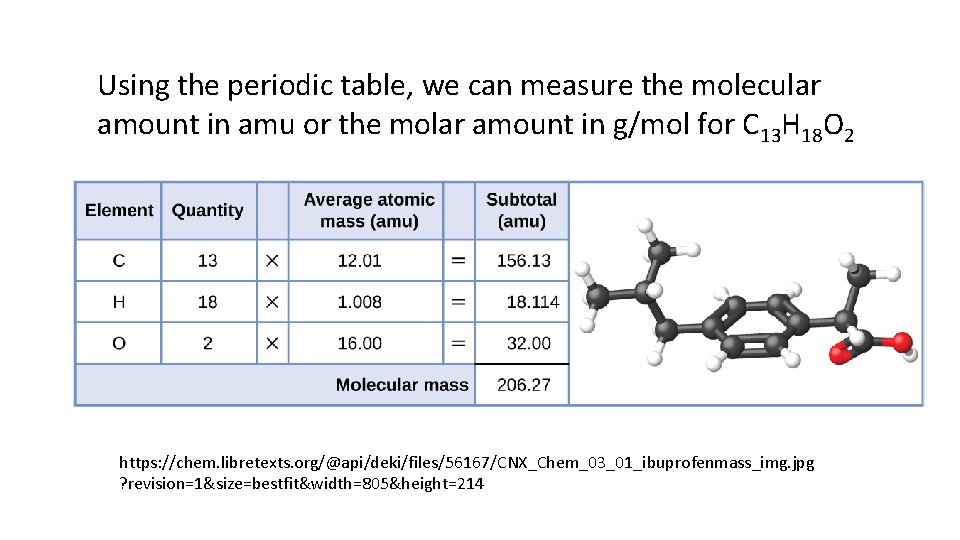
Using the periodic table, we can measure the molecular amount in amu or the molar amount in g/mol for C 13 H 18 O 2 https: //chem. libretexts. org/@api/deki/files/56167/CNX_Chem_03_01_ibuprofenmass_img. jpg ? revision=1&size=bestfit&width=805&height=214

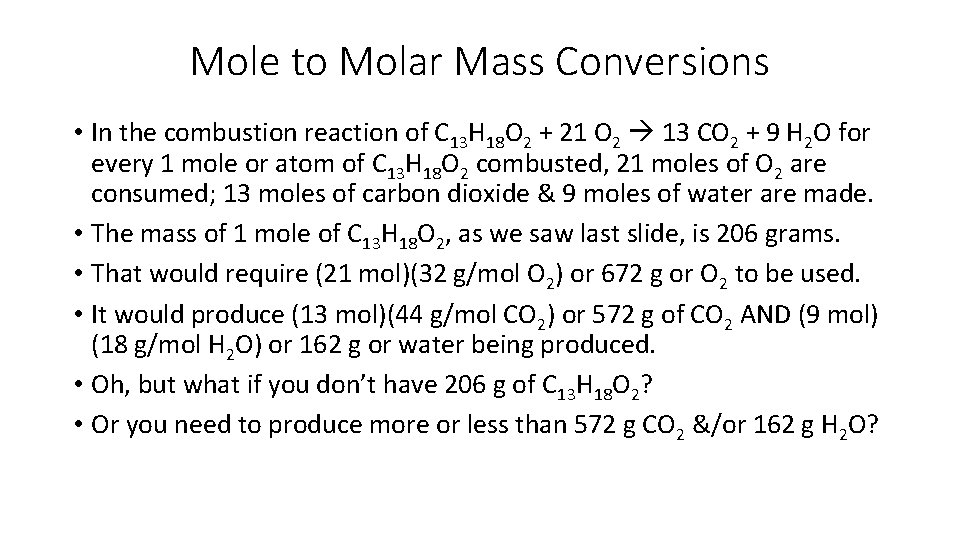
Mole to Molar Mass Conversions • In the combustion reaction of C 13 H 18 O 2 + 21 O 2 13 CO 2 + 9 H 2 O for every 1 mole or atom of C 13 H 18 O 2 combusted, 21 moles of O 2 are consumed; 13 moles of carbon dioxide & 9 moles of water are made. • The mass of 1 mole of C 13 H 18 O 2, as we saw last slide, is 206 grams. • That would require (21 mol)(32 g/mol O 2) or 672 g or O 2 to be used. • It would produce (13 mol)(44 g/mol CO 2) or 572 g of CO 2 AND (9 mol) (18 g/mol H 2 O) or 162 g or water being produced. • Oh, but what if you don’t have 206 g of C 13 H 18 O 2? • Or you need to produce more or less than 572 g CO 2 &/or 162 g H 2 O?

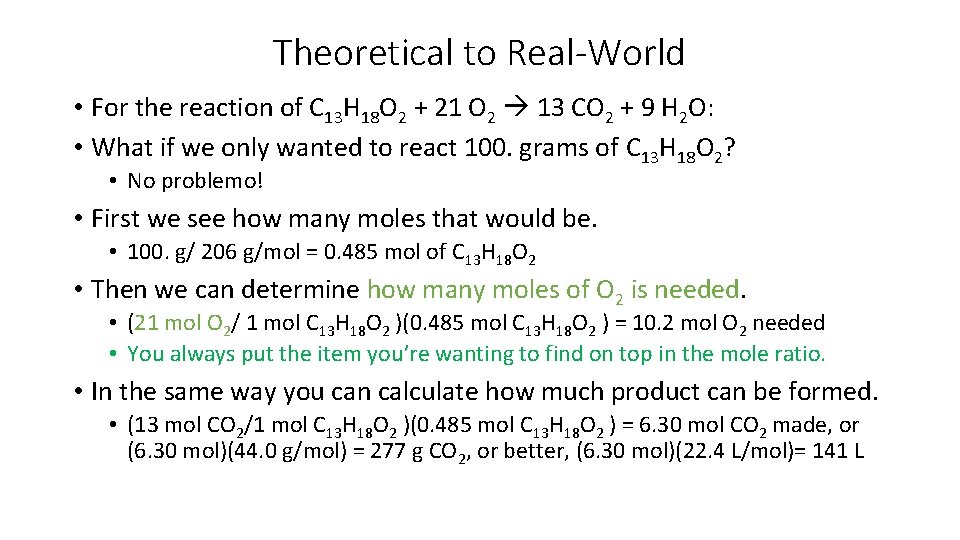
Theoretical to Real-World • For the reaction of C 13 H 18 O 2 + 21 O 2 13 CO 2 + 9 H 2 O: • What if we only wanted to react 100. grams of C 13 H 18 O 2? • No problemo! • First we see how many moles that would be. • 100. g/ 206 g/mol = 0. 485 mol of C 13 H 18 O 2 • Then we can determine how many moles of O 2 is needed. • (21 mol O 2/ 1 mol C 13 H 18 O 2 )(0. 485 mol C 13 H 18 O 2 ) = 10. 2 mol O 2 needed • You always put the item you’re wanting to find on top in the mole ratio. • In the same way you can calculate how much product can be formed. • (13 mol CO 2/1 mol C 13 H 18 O 2 )(0. 485 mol C 13 H 18 O 2 ) = 6. 30 mol CO 2 made, or (6. 30 mol)(44. 0 g/mol) = 277 g CO 2, or better, (6. 30 mol)(22. 4 L/mol)= 141 L

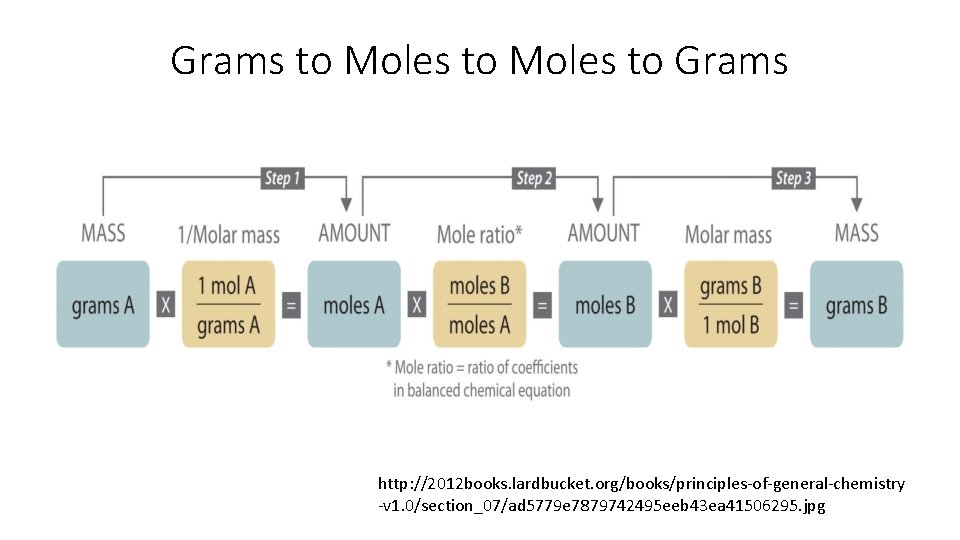
Grams to Moles to Grams http: //2012 books. lardbucket. org/books/principles-of-general-chemistry -v 1. 0/section_07/ad 5779 e 7879742495 eeb 43 ea 41506295. jpg

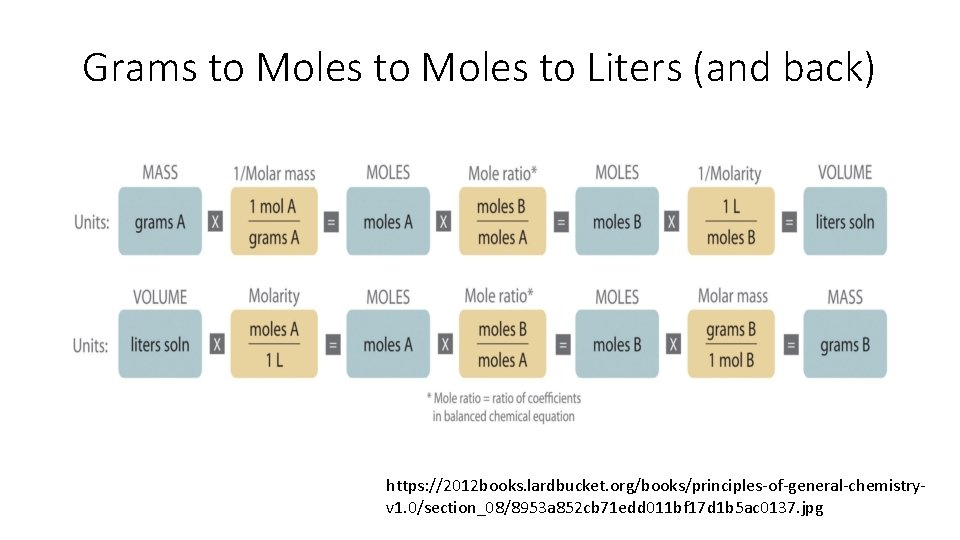
Grams to Moles to Liters (and back) https: //2012 books. lardbucket. org/books/principles-of-general-chemistryv 1. 0/section_08/8953 a 852 cb 71 edd 011 bf 17 d 1 b 5 ac 0137. jpg

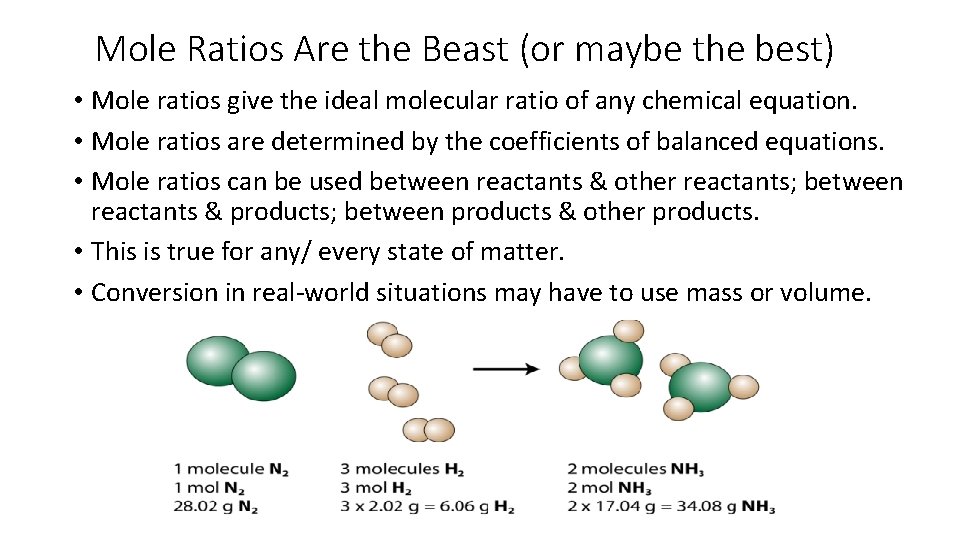
Mole Ratios Are the Beast (or maybe the best) • Mole ratios give the ideal molecular ratio of any chemical equation. • Mole ratios are determined by the coefficients of balanced equations. • Mole ratios can be used between reactants & other reactants; between reactants & products; between products & other products. • This is true for any/ every state of matter. • Conversion in real-world situations may have to use mass or volume.

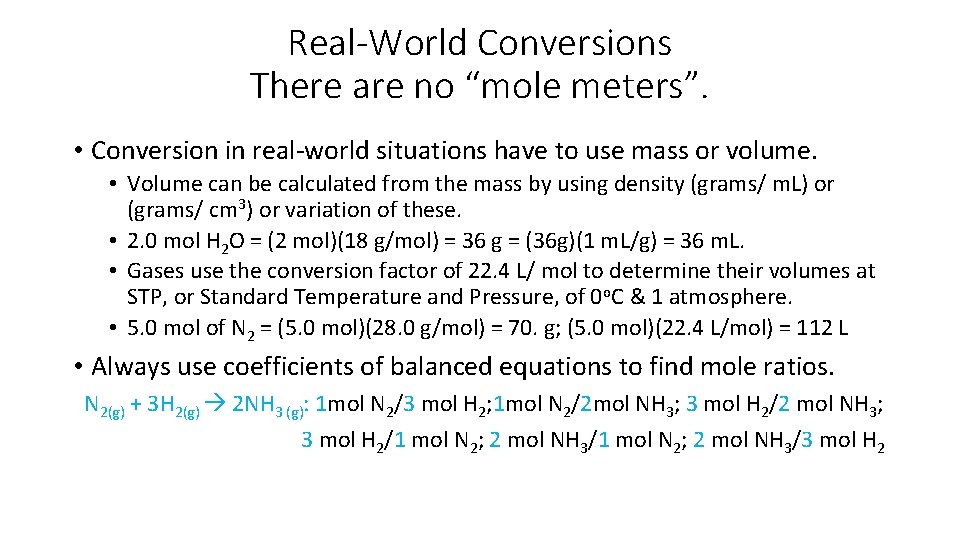
Real-World Conversions There are no “mole meters”. • Conversion in real-world situations have to use mass or volume. • Volume can be calculated from the mass by using density (grams/ m. L) or (grams/ cm 3) or variation of these. • 2. 0 mol H 2 O = (2 mol)(18 g/mol) = 36 g = (36 g)(1 m. L/g) = 36 m. L. • Gases use the conversion factor of 22. 4 L/ mol to determine their volumes at STP, or Standard Temperature and Pressure, of 0 o. C & 1 atmosphere. • 5. 0 mol of N 2 = (5. 0 mol)(28. 0 g/mol) = 70. g; (5. 0 mol)(22. 4 L/mol) = 112 L • Always use coefficients of balanced equations to find mole ratios. N 2(g) + 3 H 2(g) 2 NH 3 (g): 1 mol N 2/3 mol H 2; 1 mol N 2/2 mol NH 3; 3 mol H 2/2 mol NH 3; 3 mol H 2/1 mol N 2; 2 mol NH 3/3 mol H 2

The Trick is Setting Up Mole Ratios • You will always be given a certain quantity (mole, gram, m. L, …) of one or more of the substances in a chemical reaction => actual # moles. • When you want to find the amount of a different substance in the reaction, you make a mole ratio of the given & the unknown from the balanced equation. • You always put the moles of the unknown, what you want to find, on the top of your mole ratio. Remember – this is from the balanced equation. • You will multiply the moles of the given substance by the mole ratio that you made using coefficients of the balanced equation. • In this way, you cancel out what you were given & find # moles of the desired. • (# mol of unknown/ # mol of given)(actual # mole of given) = actual # mole of the unknown, what is desired to know.

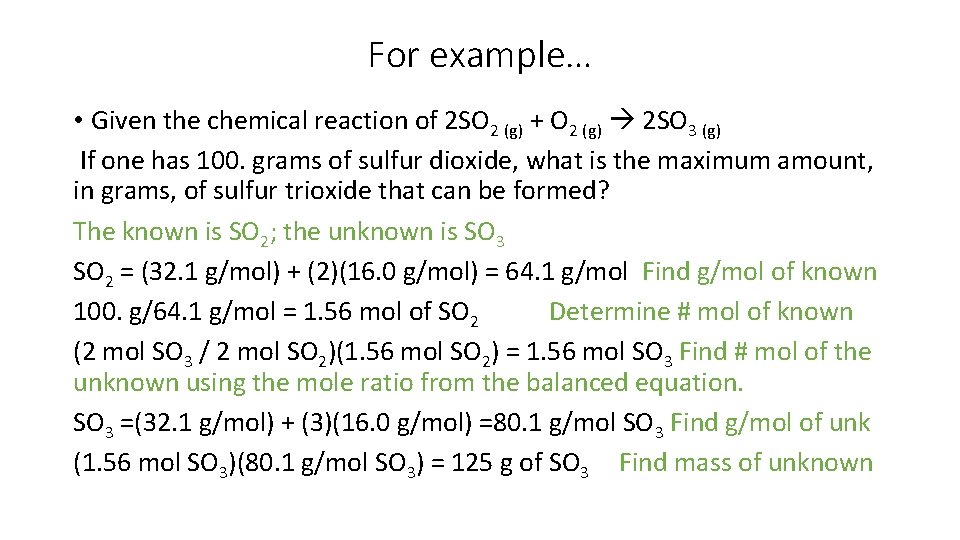
For example… • Given the chemical reaction of 2 SO 2 (g) + O 2 (g) 2 SO 3 (g) If one has 100. grams of sulfur dioxide, what is the maximum amount, in grams, of sulfur trioxide that can be formed? The known is SO 2; the unknown is SO 3 SO 2 = (32. 1 g/mol) + (2)(16. 0 g/mol) = 64. 1 g/mol Find g/mol of known 100. g/64. 1 g/mol = 1. 56 mol of SO 2 Determine # mol of known (2 mol SO 3 / 2 mol SO 2)(1. 56 mol SO 2) = 1. 56 mol SO 3 Find # mol of the unknown using the mole ratio from the balanced equation. SO 3 =(32. 1 g/mol) + (3)(16. 0 g/mol) =80. 1 g/mol SO 3 Find g/mol of unk (1. 56 mol SO 3)(80. 1 g/mol SO 3) = 125 g of SO 3 Find mass of unknown

It’s like adjusting a recipe for more/less • DIY Hand sanitizer (from the Internet) https: //myheavenlyrecipes. com/how-tomake-your-own-hand-sanitizer/ • ⅔ Cup 99% Rubbing Alcohol • ⅓ Cup Aloe Vera Gel • Essential Oils Of Your Choice (5 drops? ) • But you want to make enough for all your relative, as in 25 batches. • (25 batches)(0. 66 cups alcohol/ batch) = 16. 67 cups of alcohol • (25 batches)(0. 33 cups Aloe Vera gel/ batch) = 8. 33 cups Aloe Vera gel • (25 batches)(5 drop/ batch)/ (20 drops/ 1 m. L) = 6. 25 m. L of choice oil

Or if you didn’t want that much…. • DIY Hand sanitizer (from the Internet) https: //myheavenlyrecipes. com/how-tomake-your-own-hand-sanitizer/ • ⅔ Cup 99% Rubbing Alcohol (ROH) • ⅓ Cup Aloe Vera Gel (AVG) • Essential Oils Of Your Choice (5 drops? ) (EO) • Desire only 0. 5 cup final product: Need to downsize. • (0. 66 cup ROH/ 1. 0 cup sanitizer)(0. 5 cup sanitizer) = 0. 33 cup ROH • (0. 33 cup AVG/ 1. 0 cup sanitizer)(0. 5 cup sanitizer) = 0. 16 cup AVG • (5 drops EO/ 1. 0 cup sanitizer)(0. 5 cup sanitizer) = 2 - 3 drops EO
 Chapter 9 modern chemistry review answers
Chapter 9 modern chemistry review answers Zeus
Zeus Amanda rinehart
Amanda rinehart Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Chapter 11 stoichiometry assessment answer key
Chapter 11 stoichiometry assessment answer key Stoichiometry chapter 9 test
Stoichiometry chapter 9 test Holt physics chapter 16 test
Holt physics chapter 16 test Holt physics chapter 8 fluid mechanics test answers
Holt physics chapter 8 fluid mechanics test answers A man named bungkas climbed a palm tree
A man named bungkas climbed a palm tree Holt geometry chapter 5
Holt geometry chapter 5 Chemistry regents january 2018 answers
Chemistry regents january 2018 answers Ap chemistry stoichiometry
Ap chemistry stoichiometry Stoichiometry chemistry definition
Stoichiometry chemistry definition General chemistry 1 stoichiometry
General chemistry 1 stoichiometry Ap chemistry stoichiometry
Ap chemistry stoichiometry