Stoichiometry Chapter 9 Stoichiometry that portion of chemistry





























- Slides: 74

Stoichiometry Chapter 9

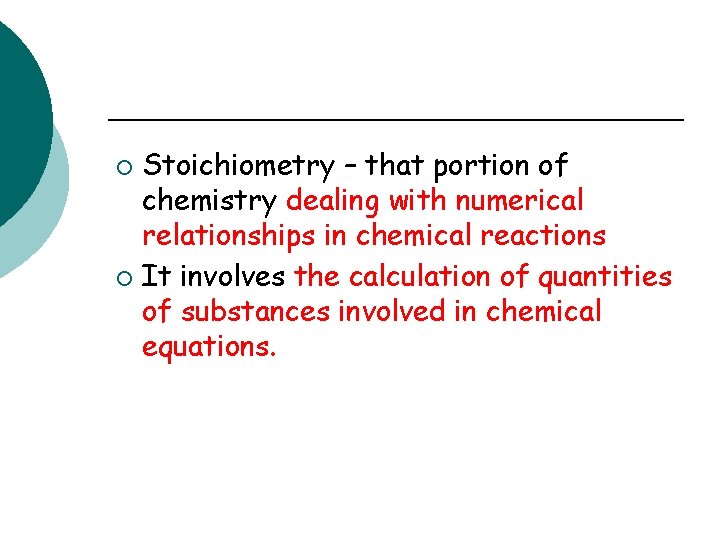
Stoichiometry – that portion of chemistry dealing with numerical relationships in chemical reactions ¡ It involves the calculation of quantities of substances involved in chemical equations. ¡

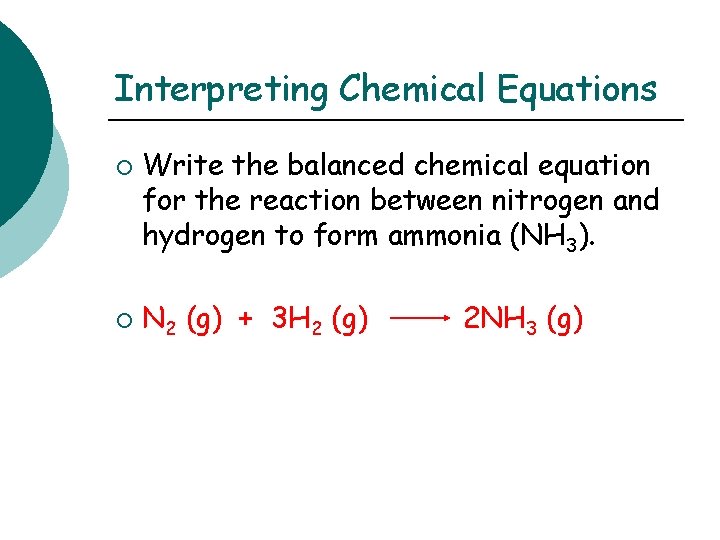
Interpreting Chemical Equations ¡ ¡ Write the balanced chemical equation for the reaction between nitrogen and hydrogen to form ammonia (NH 3). N 2 (g) + 3 H 2 (g) 2 NH 3 (g)

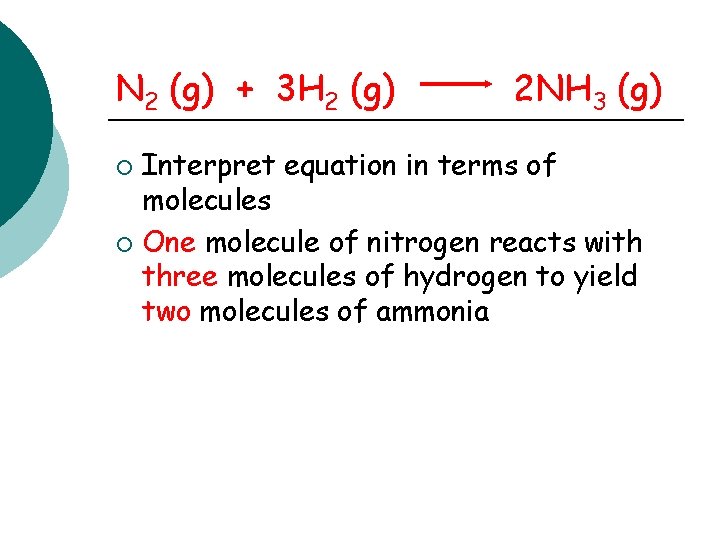
N 2 (g) + 3 H 2 (g) 2 NH 3 (g) Interpret equation in terms of molecules ¡ One molecule of nitrogen reacts with three molecules of hydrogen to yield two molecules of ammonia ¡

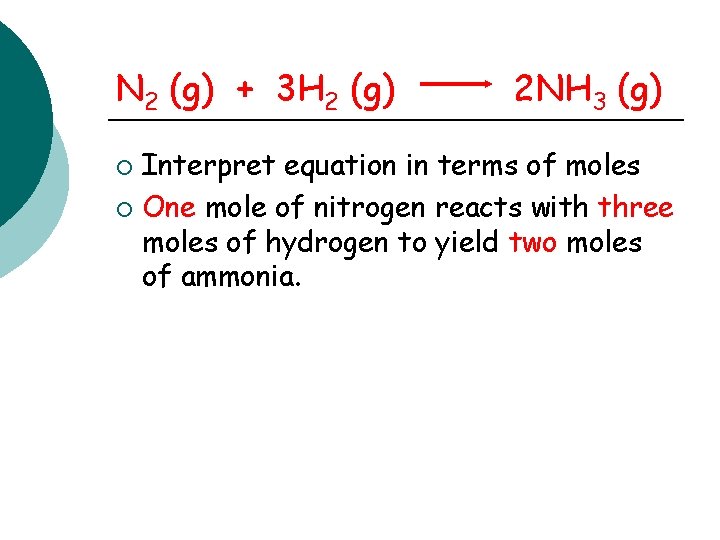
N 2 (g) + 3 H 2 (g) 2 NH 3 (g) Interpret equation in terms of moles ¡ One mole of nitrogen reacts with three moles of hydrogen to yield two moles of ammonia. ¡

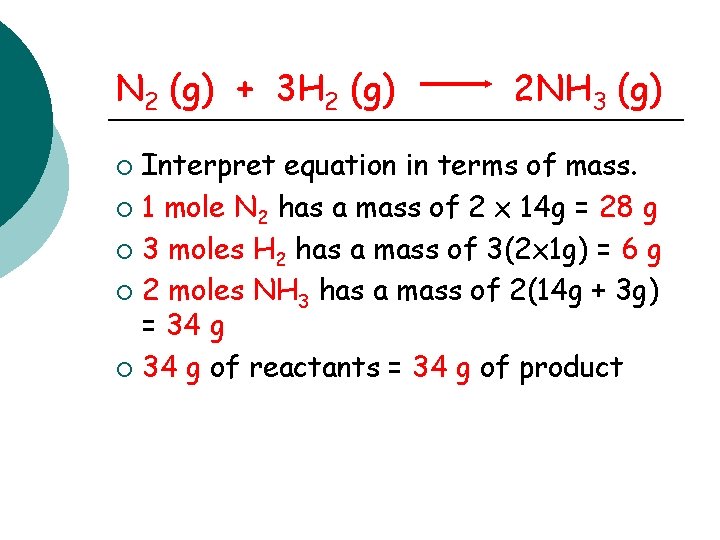
N 2 (g) + 3 H 2 (g) 2 NH 3 (g) Interpret equation in terms of mass. ¡ 1 mole N 2 has a mass of 2 x 14 g = 28 g ¡ 3 moles H 2 has a mass of 3(2 x 1 g) = 6 g ¡ 2 moles NH 3 has a mass of 2(14 g + 3 g) = 34 g ¡ 34 g of reactants = 34 g of product ¡

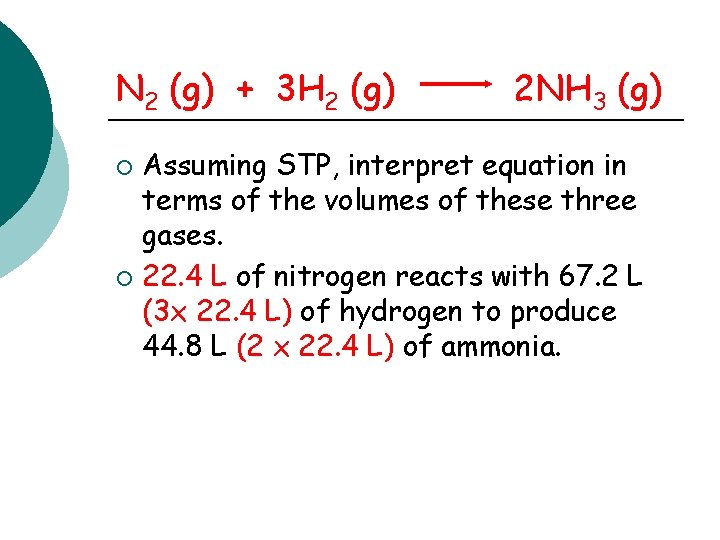
N 2 (g) + 3 H 2 (g) 2 NH 3 (g) Assuming STP, interpret equation in terms of the volumes of these three gases. ¡ 22. 4 L of nitrogen reacts with 67. 2 L (3 x 22. 4 L) of hydrogen to produce 44. 8 L (2 x 22. 4 L) of ammonia. ¡

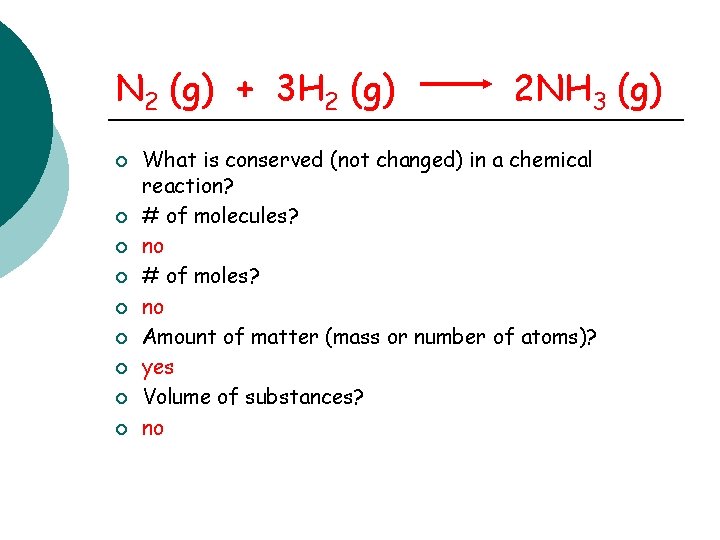
N 2 (g) + 3 H 2 (g) ¡ ¡ ¡ ¡ ¡ 2 NH 3 (g) What is conserved (not changed) in a chemical reaction? # of molecules? no # of moles? no Amount of matter (mass or number of atoms)? yes Volume of substances? no

Law of Conservation of Mass The Law of Conservation of Mass indicates that in an ordinary chemical reaction, Matter cannot be created or destroyed. No change in total mass occurs in a reaction. Mass of products is equal to mass of reactants. 9

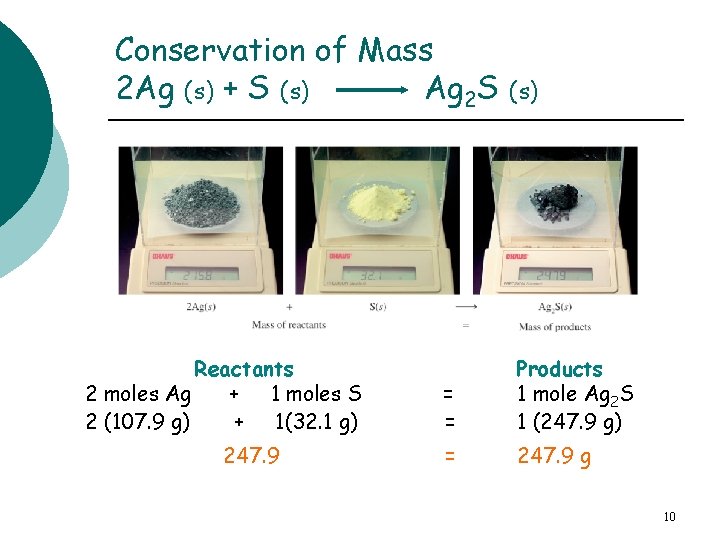
Conservation of Mass 2 Ag (s) + S (s) Ag 2 S (s) Reactants 2 moles Ag + 1 moles S 2 (107. 9 g) + 1(32. 1 g) 247. 9 = = Products 1 mole Ag 2 S 1 (247. 9 g) = 247. 9 g 10

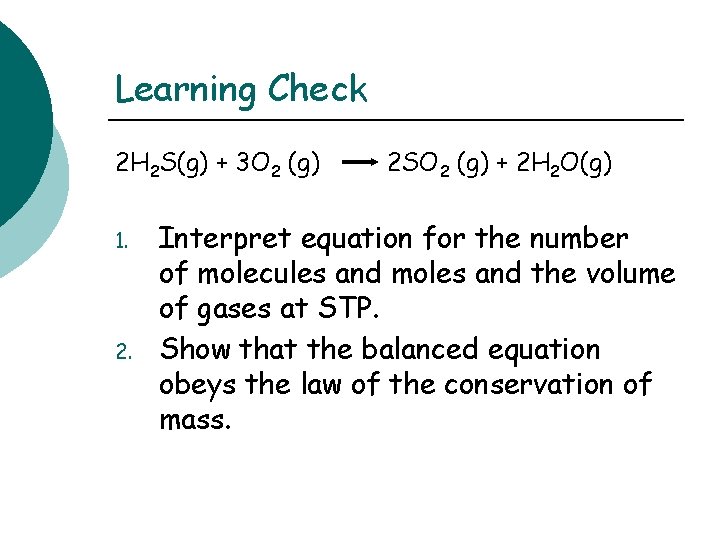
Learning Check 2 H 2 S(g) + 3 O 2 (g) 1. 2 SO 2 (g) + 2 H 2 O(g) Interpret equation for the number of molecules and moles and the volume of gases at STP. Show that the balanced equation obeys the law of the conservation of mass.

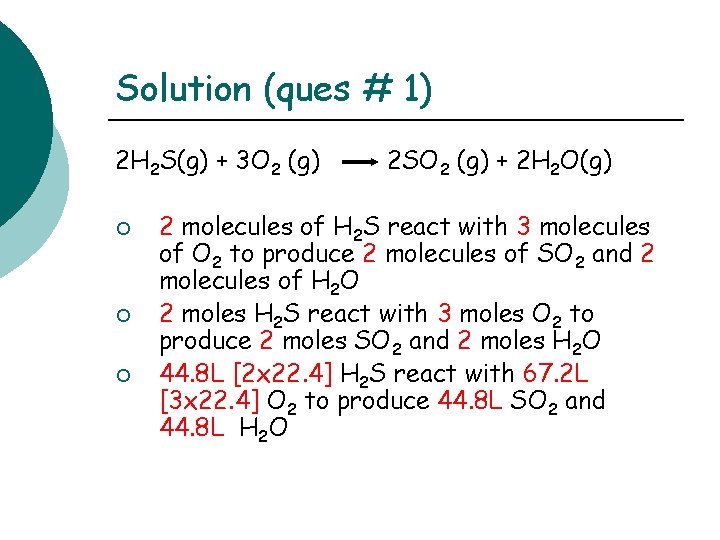
Solution (ques # 1) 2 H 2 S(g) + 3 O 2 (g) ¡ ¡ ¡ 2 SO 2 (g) + 2 H 2 O(g) 2 molecules of H 2 S react with 3 molecules of O 2 to produce 2 molecules of SO 2 and 2 molecules of H 2 O 2 moles H 2 S react with 3 moles O 2 to produce 2 moles SO 2 and 2 moles H 2 O 44. 8 L [2 x 22. 4] H 2 S react with 67. 2 L [3 x 22. 4] O 2 to produce 44. 8 L SO 2 and 44. 8 L H 2 O

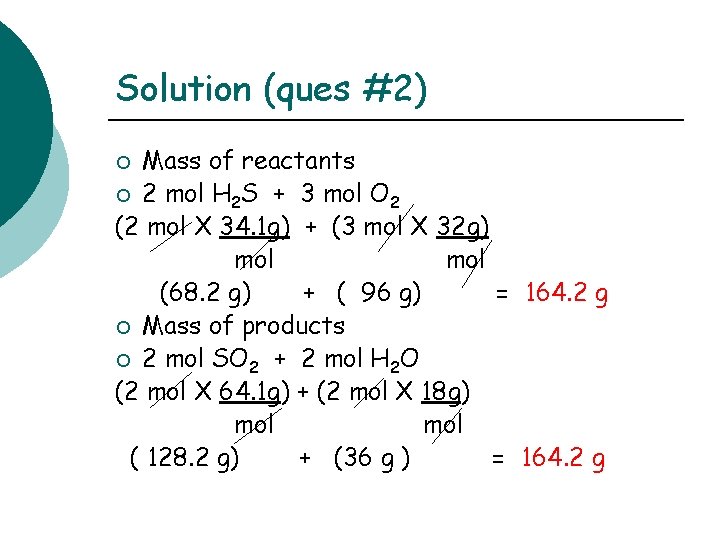
Solution (ques #2) Mass of reactants ¡ 2 mol H 2 S + 3 mol O 2 (2 mol X 34. 1 g) + (3 mol X 32 g) mol (68. 2 g) + ( 96 g) = 164. 2 g ¡ Mass of products ¡ 2 mol SO 2 + 2 mol H 2 O (2 mol X 64. 1 g) + (2 mol X 18 g) mol ( 128. 2 g) + (36 g ) = 164. 2 g ¡

Sample problems (grams to moles and vice-versa) ¡ How many moles of ice melt (Mg. Cl 2) do we have in a 10 lb bag l (10 ¡ How lbs is 4, 535 grams)? many grams are in. 2 moles of Mg. Cl 2

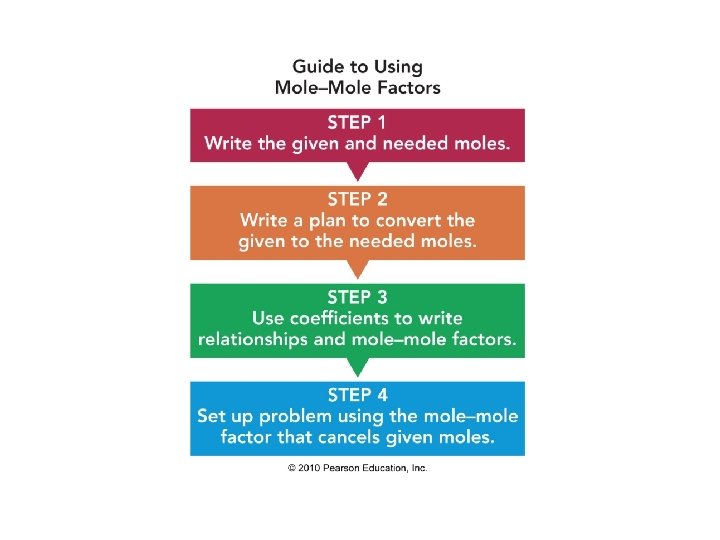
A mole–mole factor is a ratio of the moles (from the coefficients) for any two substances in an equation

4 Fe(s) + 3 O 2(g) Fe and O 2: Fe and Fe 2 O 3: O 2 and Fe 2 O 3: 2 Fe 2 O 3(s) 4 moles Fe and 3 moles O 2 4 moles Fe and 2 moles Fe 2 O 3 3 moles O 2 4 moles Fe 2 O 3 3 moles O 2

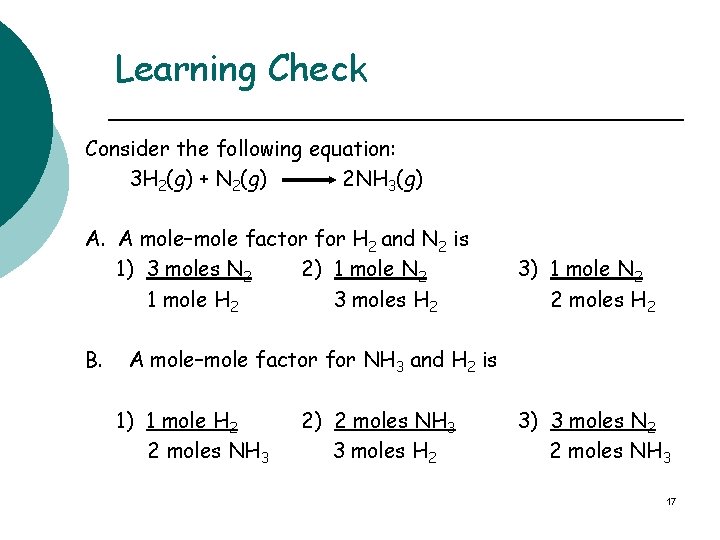
Learning Check Consider the following equation: 3 H 2(g) + N 2(g) 2 NH 3(g) A. A mole–mole factor for H 2 and N 2 is 1) 3 moles N 2 2) 1 mole N 2 1 mole H 2 3 moles H 2 B. 3) 1 mole N 2 2 moles H 2 A mole–mole factor for NH 3 and H 2 is 1) 1 mole H 2 2 moles NH 3 2) 2 moles NH 3 3 moles H 2 3) 3 moles N 2 2 moles NH 3 17

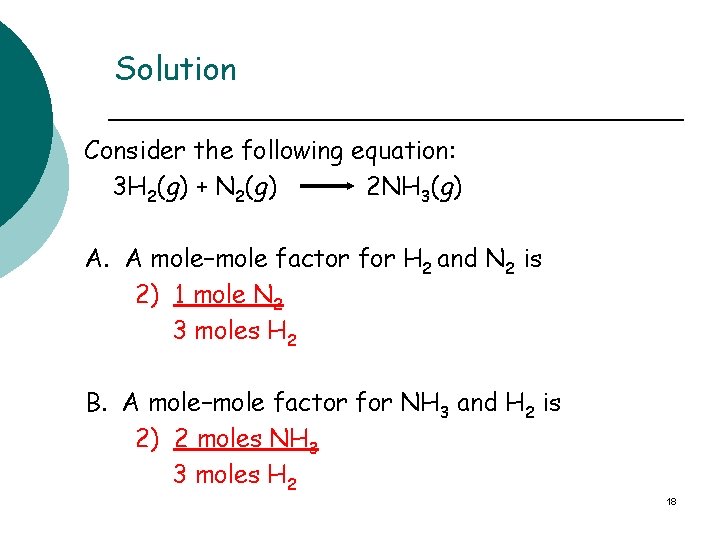
Solution Consider the following equation: 3 H 2(g) + N 2(g) 2 NH 3(g) A. A mole–mole factor for H 2 and N 2 is 2) 1 mole N 2 3 moles H 2 B. A mole–mole factor for NH 3 and H 2 is 2) 2 moles NH 3 3 moles H 2 18


Given: 4 Fe(s) + 3 O 2(g) 2 Fe 2 O 3(s) How many moles of Fe are needed for the reaction of 12. 0 moles of O 2? 20

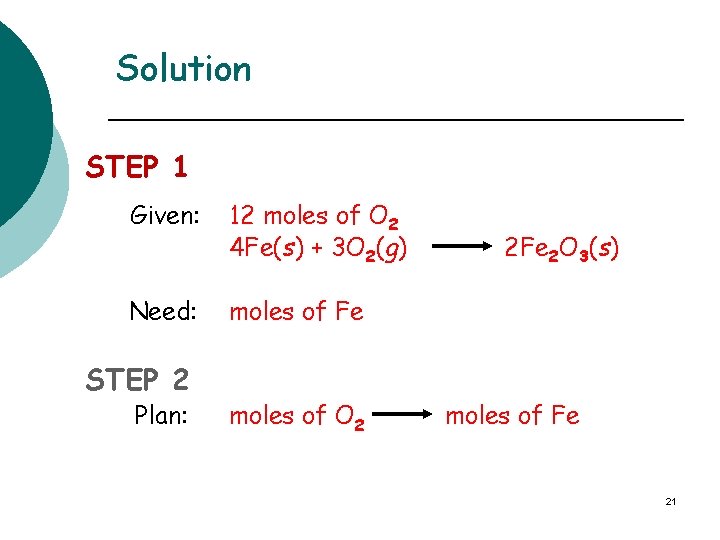
Solution STEP 1 Given: 12 moles of O 2 4 Fe(s) + 3 O 2(g) Need: moles of Fe STEP 2 Plan: moles of O 2 2 Fe 2 O 3(s) moles of Fe 21

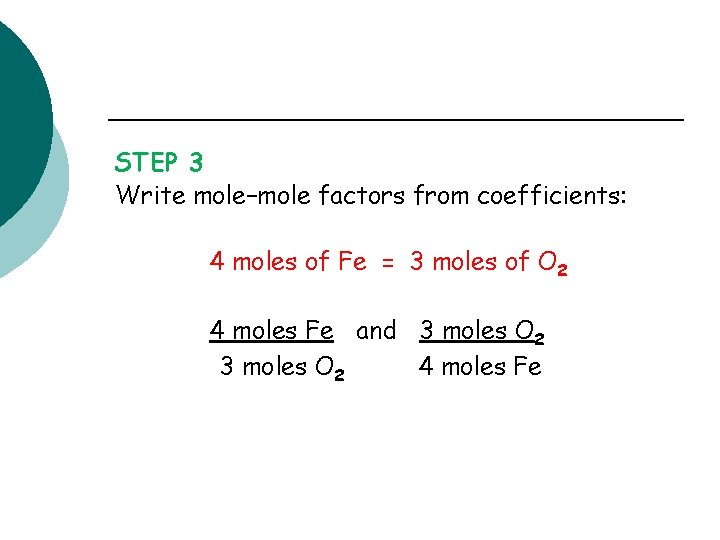
STEP 3 Write mole–mole factors from coefficients: 4 moles of Fe = 3 moles of O 2 4 moles Fe and 3 moles O 2 4 moles Fe

Solution (continued) STEP 4 Set up problem to cancel moles of O 2: 12. 0 mol O 2 x 4 mol Fe = 16. 0 moles of Fe 3 moles O 2 23

Assignment ¡ Sample Problem 9 -3 ¡ Basic Stoichiometry (Mass to Mass l 1 a&b

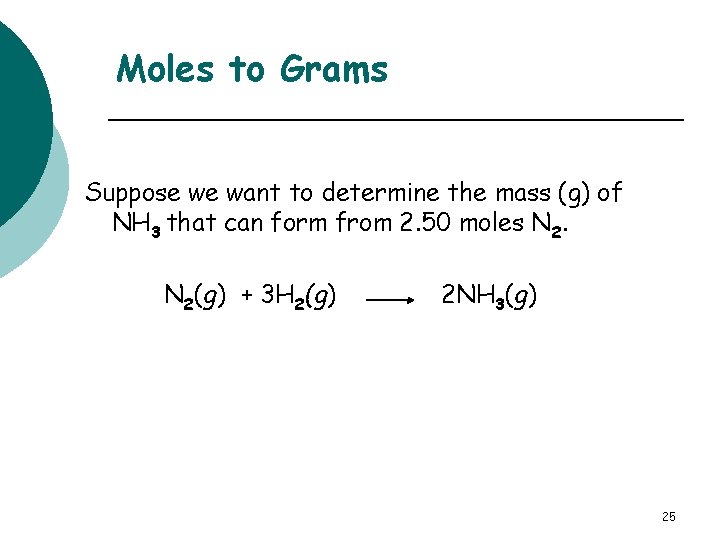
Moles to Grams Suppose we want to determine the mass (g) of NH 3 that can form from 2. 50 moles N 2(g) + 3 H 2(g) 2 NH 3(g) 25

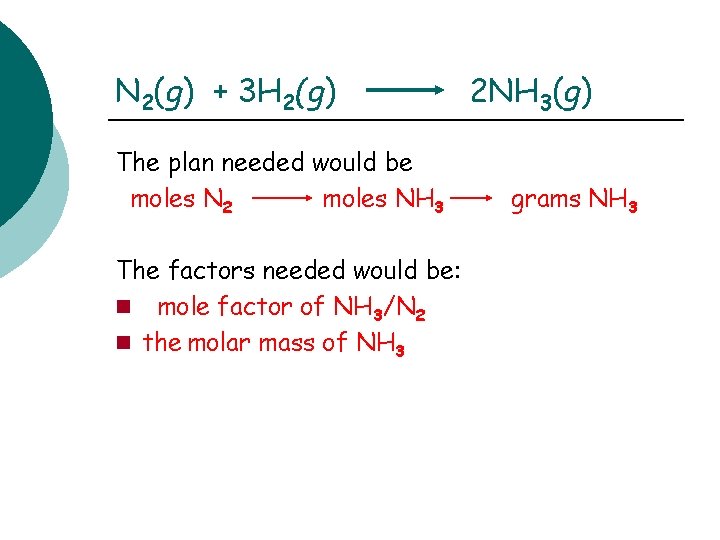
N 2(g) + 3 H 2(g) The plan needed would be moles N 2 moles NH 3 The factors needed would be: mole factor of NH 3/N 2 the molar mass of NH 3 2 NH 3(g) grams NH 3

N 2(g) + 3 H 2(g) 2 NH 3(g) The setup for the solution would be: 2. 50 mole N 2 x 2 moles NH 3 x 17. 0 g NH 3 1 mole N 2 1 mole NH 3 given mole-mole factor molar mass = 85. 0 g NH 3 27

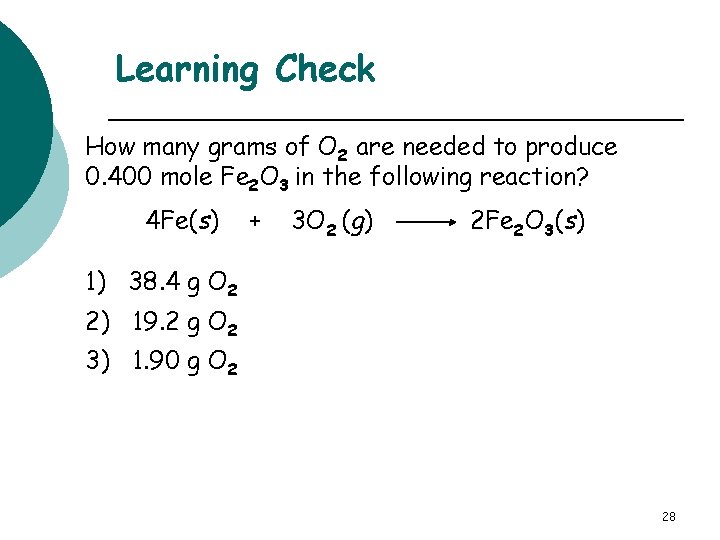
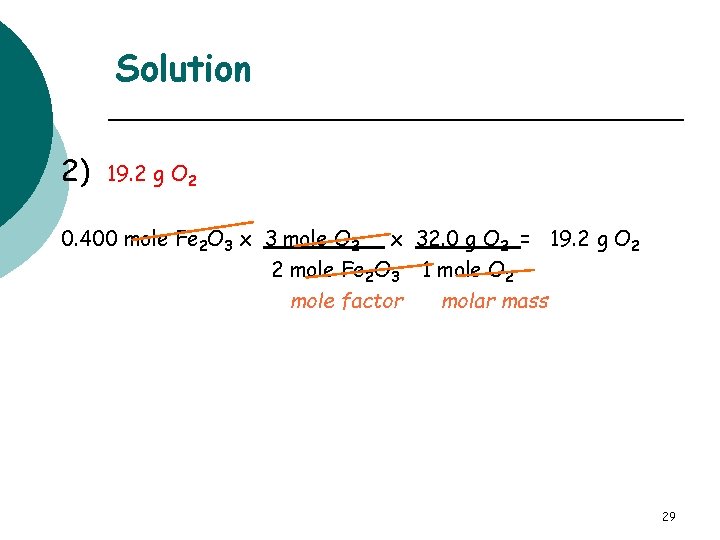
Learning Check How many grams of O 2 are needed to produce 0. 400 mole Fe 2 O 3 in the following reaction? 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O 3(s) 1) 38. 4 g O 2 2) 19. 2 g O 2 3) 1. 90 g O 2 28

Solution 2) 19. 2 g O 2 0. 400 mole Fe 2 O 3 x 3 mole O 2 x 32. 0 g O 2 = 19. 2 g O 2 2 mole Fe 2 O 3 1 mole O 2 mole factor molar mass 29

Mass to Mass Calculations You can not use a balanced equation to obtain a direct mass to mass relationship. ¡ Change the known mass to moles using molecular mass conversion factor ( molecular mass of a substance in grams = 1 mole) ¡

Then use the coefficients in the equation to determine the mole to mole factor between the known mass and the unknown mass. ¡ Once you know the moles of the unknown you can then use the molar mass of the unknown to convert moles to grams. ¡

Steps 1. 2. 3. Change known mass to moles using molar mass Determine moles of unknown by using the coefficients of the equation to set up a mole-mole factor Once the moles of unknown is determined, use its molar mass to determine mass of unknown.

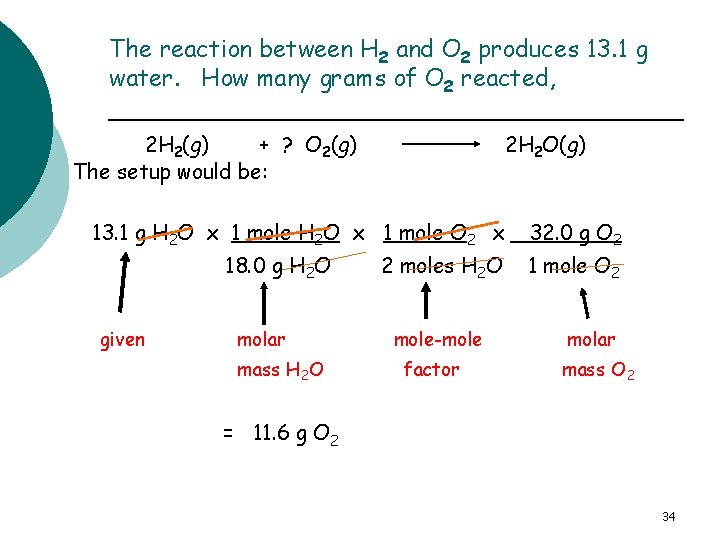
Mass of a Reactant The reaction between H 2 and O 2 produces 13. 1 g water. How many grams of O 2 reacted? 2 H 2(g) + ? O 2(g) 2 H 2 O(g) The plan and factors would be g H 2 O mole H 2 O molar mass H 2 O mole O 2 mole-mole factor g O 2 molar mass O 2 33

The reaction between H 2 and O 2 produces 13. 1 g water. How many grams of O 2 reacted, 2 H 2(g) + ? O 2(g) The setup would be: 2 H 2 O(g) 13. 1 g H 2 O x 1 mole O 2 x 18. 0 g H 2 O given molar mass H 2 O 2 moles H 2 O mole-mole factor 32. 0 g O 2 1 mole O 2 molar mass O 2 = 11. 6 g O 2 34

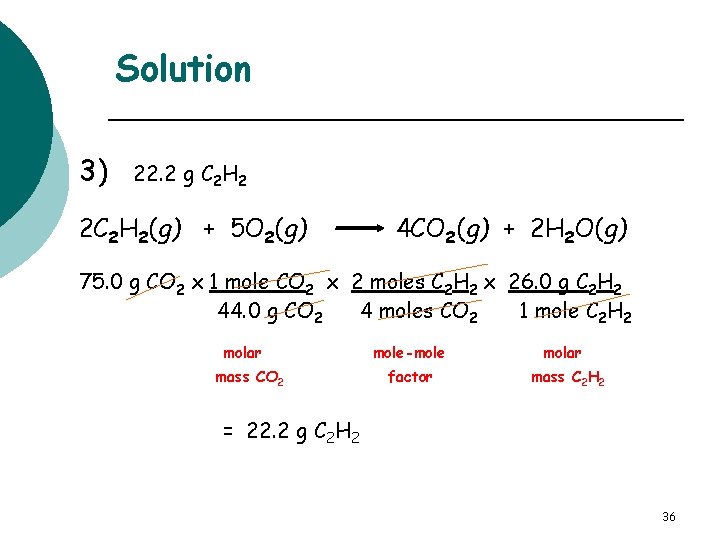
Learning Check Acetylene gas C 2 H 2 burns in the oxyacetylene torch for welding. How many grams of C 2 H 2 are burned if the reaction produces 75. 0 g CO 2? 2 C 2 H 2(g) + 5 O 2(g) 4 CO 2(g) + 2 H 2 O(g) 1) 88. 6 g C 2 H 2 2) 44. 3 g C 2 H 2 3) 22. 2 g C 2 H 2 35

Solution 3) 22. 2 g C 2 H 2 2 C 2 H 2(g) + 5 O 2(g) 4 CO 2(g) + 2 H 2 O(g) 75. 0 g CO 2 x 1 mole CO 2 x 2 moles C 2 H 2 x 26. 0 g C 2 H 2 44. 0 g CO 2 4 moles CO 2 1 mole C 2 H 2 molar mass CO 2 mole-mole factor molar mass C 2 H 2 = 22. 2 g C 2 H 2 36

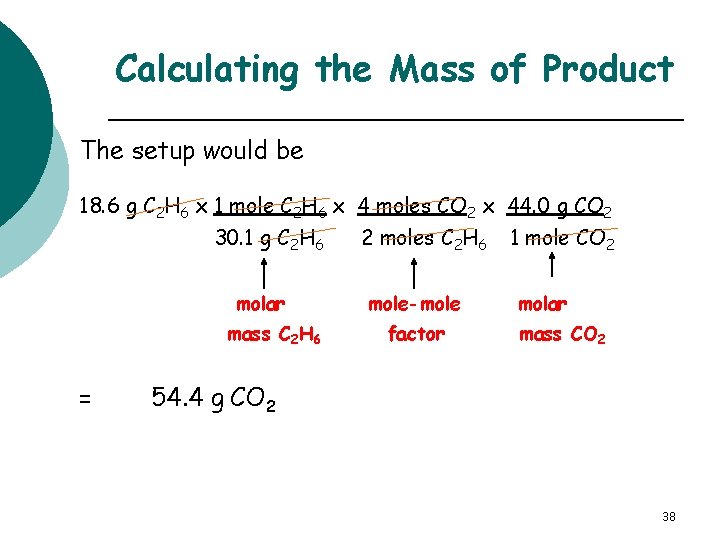
Mass of Product When 18. 6 g ethane gas C 2 H 6 burns in oxygen, how many grams of CO 2 are produced? 2 C 2 H 6(g) + 7 O 2(g) 18. 6 g 4 CO 2(g) + 6 H 2 O(g) ? g The plan and factors would be g C 2 H 6 mole C 2 H 6 mole CO 2 g CO 2 molar mass C 2 H 6 mole-mole factor molar mass CO 2 37

Calculating the Mass of Product The setup would be 18. 6 g C 2 H 6 x 1 mole C 2 H 6 x 4 moles CO 2 x 44. 0 g CO 2 30. 1 g C 2 H 6 molar mass C 2 H 6 = 2 moles C 2 H 6 mole-mole factor 1 mole CO 2 molar mass CO 2 54. 4 g CO 2 38

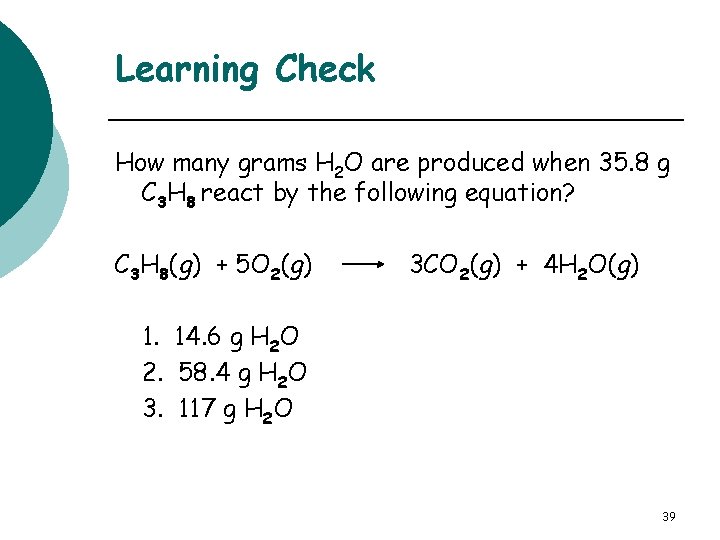
Learning Check How many grams H 2 O are produced when 35. 8 g C 3 H 8 react by the following equation? C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g) 1. 14. 6 g H 2 O 2. 58. 4 g H 2 O 3. 117 g H 2 O 39

Solution C 3 H 8(g) + 5 O 2(g) 35. 8 g C 3 H 8 x 1 mole C 3 H 8 44. 1 g C 3 H 8 molar mass C 3 H 8 3 CO 2(g) + 4 H 2 O(g) x 4 mole H 2 O x 18. 0 g H 2 O 1 mole C 3 H 8 1 mole H 2 O mole-mole factor molar mass H 2 O = 58. 4 g H 2 O (2) 40

Limiting Reactants and Percent Yield Chapter 9 section 3

Theoretical, Actual, and Percent Yield theoretical yield - the maximum amount of product, which is calculated using the balanced equation. actual yield - the amount of product obtained when the reaction takes place 42

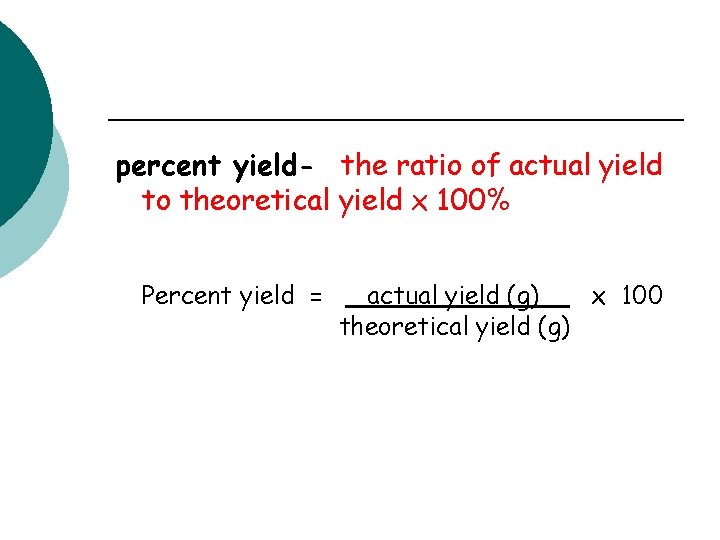
percent yield- the ratio of actual yield to theoretical yield x 100% Percent yield = actual yield (g) x 100 theoretical yield (g)

44

Calculating Percent Yield Suppose you have prepared cookie dough to make 5 dozen cookies. The phone rings and you answer. While you talk, a sheet of 12 cookies burns, and you have to throw them out. The rest of the cookies you make are okay. What is the percent yield of edible cookies? Theoretical yield: 60 cookies possible Actual yield: 48 cookies to eat Percent yield: 48 cookies x 100% = 80. %yield 60 cookies 45

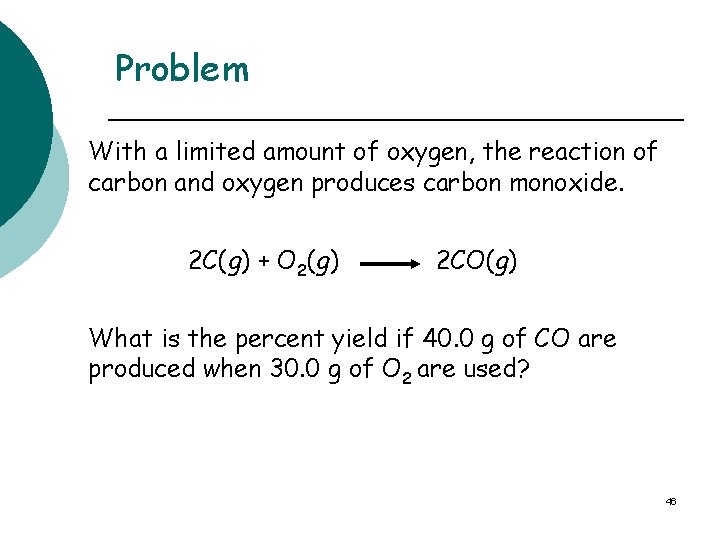
Problem With a limited amount of oxygen, the reaction of carbon and oxygen produces carbon monoxide. 2 C(g) + O 2(g) 2 CO(g) What is the percent yield if 40. 0 g of CO are produced when 30. 0 g of O 2 are used? 46

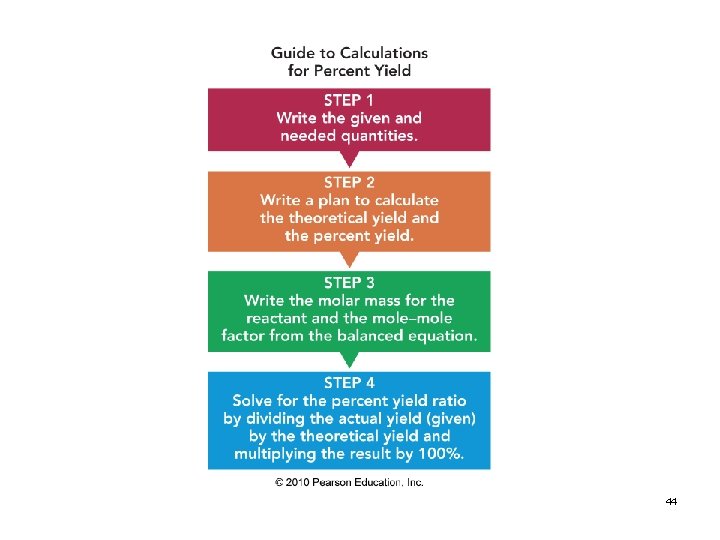
Solution STEP 1 Given: 40. 0 g of CO produced (actual) 30. 0 g of O 2 used Need: percent yield of CO 47

2 C(g) + O 2(g) 2 CO(g) STEP 2 Write a plan to calculate (theoretical) % yield of CO: g O 2 moles CO g CO

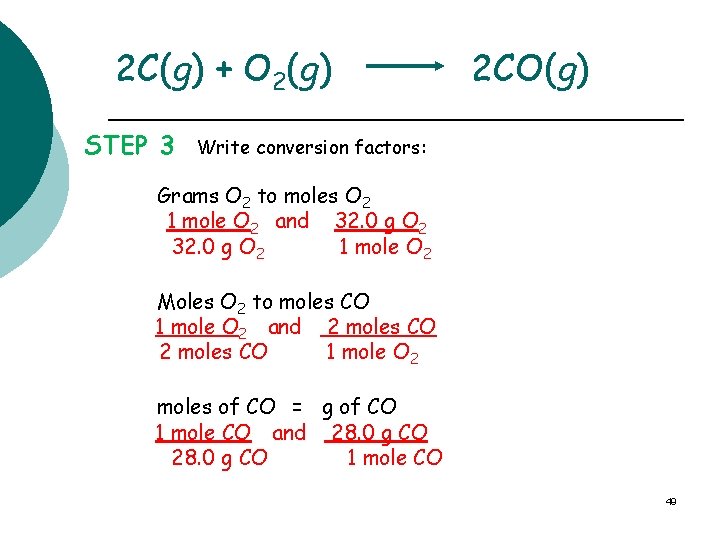
2 C(g) + O 2(g) STEP 3 2 CO(g) Write conversion factors: Grams O 2 to moles O 2 1 mole O 2 and 32. 0 g O 2 1 mole O 2 Moles O 2 to moles CO 1 mole O 2 and 2 moles CO 1 mole O 2 moles of CO = g of CO 1 mole CO and 28. 0 g CO 1 mole CO 49

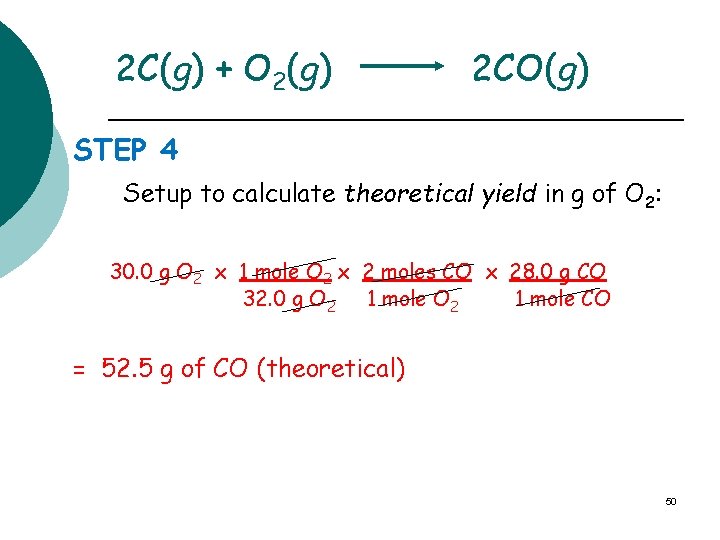
2 C(g) + O 2(g) 2 CO(g) STEP 4 Setup to calculate theoretical yield in g of O 2: 30. 0 g O 2 x 1 mole O 2 x 2 moles CO x 28. 0 g CO 32. 0 g O 2 1 mole CO = 52. 5 g of CO (theoretical) 50

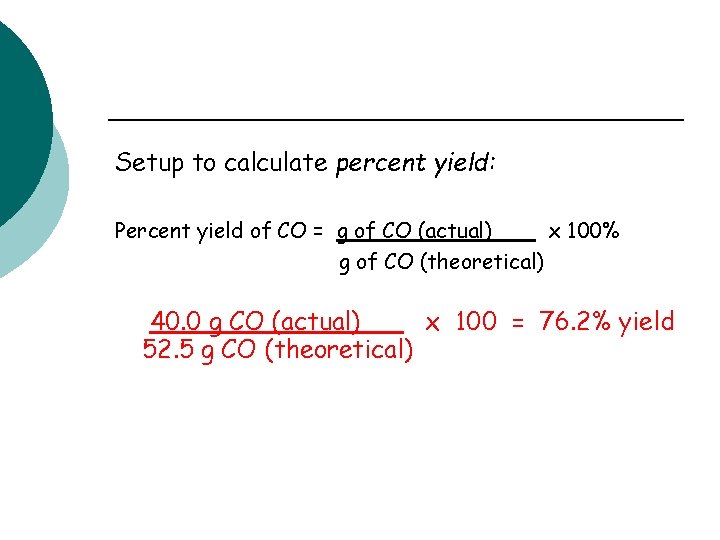
Setup to calculate percent yield: Percent yield of CO = g of CO (actual) x 100% g of CO (theoretical) 40. 0 g CO (actual) x 100 = 76. 2% yield 52. 5 g CO (theoretical)

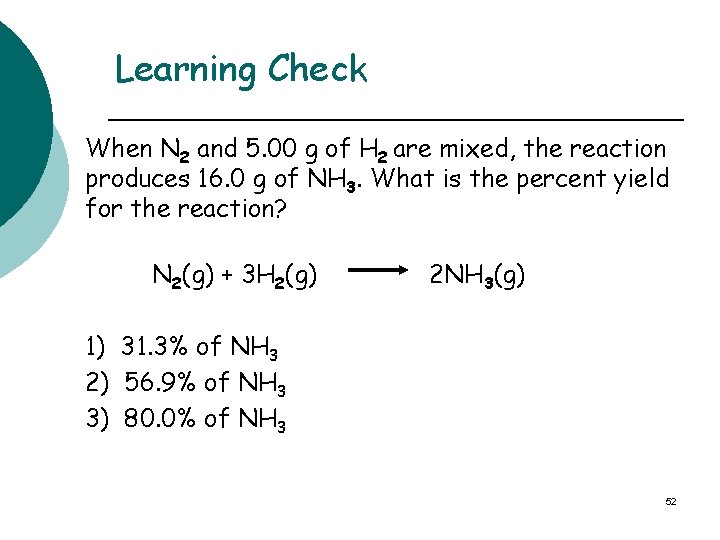
Learning Check When N 2 and 5. 00 g of H 2 are mixed, the reaction produces 16. 0 g of NH 3. What is the percent yield for the reaction? N 2(g) + 3 H 2(g) 2 NH 3(g) 1) 31. 3% of NH 3 2) 56. 9% of NH 3 3) 80. 0% of NH 3 52

Solution STEP 1 Given: 16. 0 g of NH 3 produced (actual) 5. 00 g of H 2 used Need: percent yield of NH 3 53

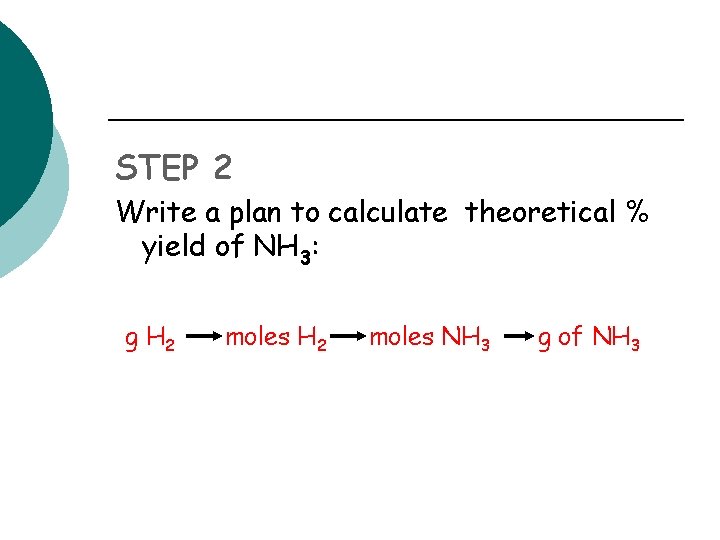
STEP 2 Write a plan to calculate theoretical % yield of NH 3: g H 2 moles NH 3 g of NH 3

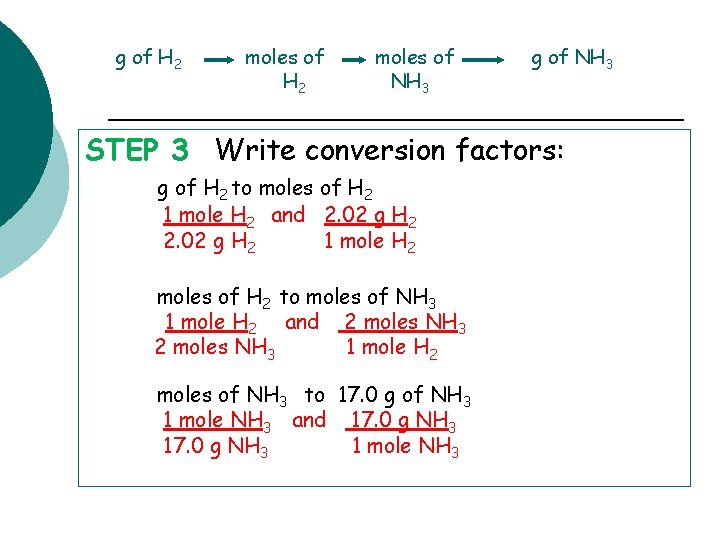
g of H 2 moles of NH 3 g of NH 3 STEP 3 Write conversion factors: g of H 2 to moles of H 2 1 mole H 2 and 2. 02 g H 2 1 mole H 2 moles of H 2 to moles of NH 3 1 mole H 2 and 2 moles NH 3 1 mole H 2 moles of NH 3 to 17. 0 g of NH 3 1 mole NH 3 and 17. 0 g NH 3 1 mole NH 3

Solution (continued) STEP 4 Setup to calculate theoretical yield of g of NH 3: 5. 00 g H 2 x 1 mole H 2 x 2 moles NH 3 x 17. 0 g NH 3 2. 02 g H 2 3 moles H 2 1 mole NH 3 = 28. 1 g of NH 3 (theoretical) 56

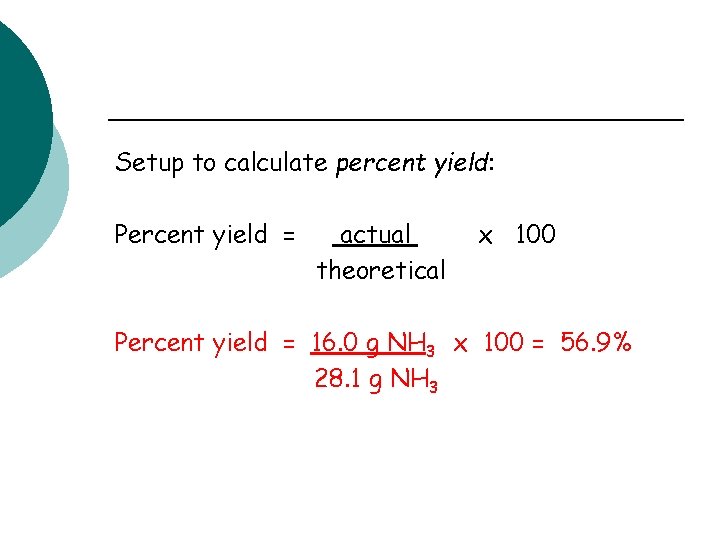
Setup to calculate percent yield: Percent yield = actual theoretical x 100 Percent yield = 16. 0 g NH 3 x 100 = 56. 9% 28. 1 g NH 3

Limiting Reactant A limiting reactant – the reactant in a chemical reaction that is used up. When the reactant is used up, the chemical reaction stops. Product stops being formed. 58

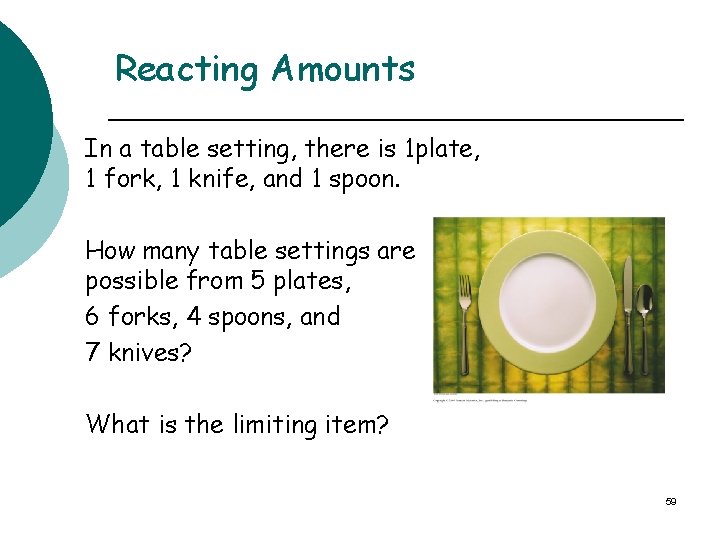
Reacting Amounts In a table setting, there is 1 plate, 1 fork, 1 knife, and 1 spoon. How many table settings are possible from 5 plates, 6 forks, 4 spoons, and 7 knives? What is the limiting item? 59

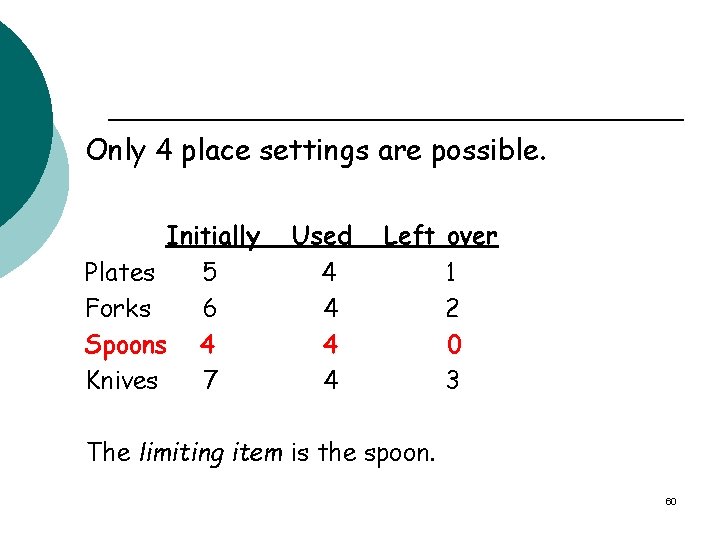
Only 4 place settings are possible. Initially Plates 5 Forks 6 Spoons 4 Knives 7 Used 4 4 Left over 1 2 0 3 The limiting item is the spoon. 60

Example 1 of an Everyday Limiting Reactant How many peanut butter sandwiches could be made from 8 slices of bread and 1 jar of peanut butter? With 8 slices of bread, only 4 sandwiches could be made. What is the limiting item? 61

62

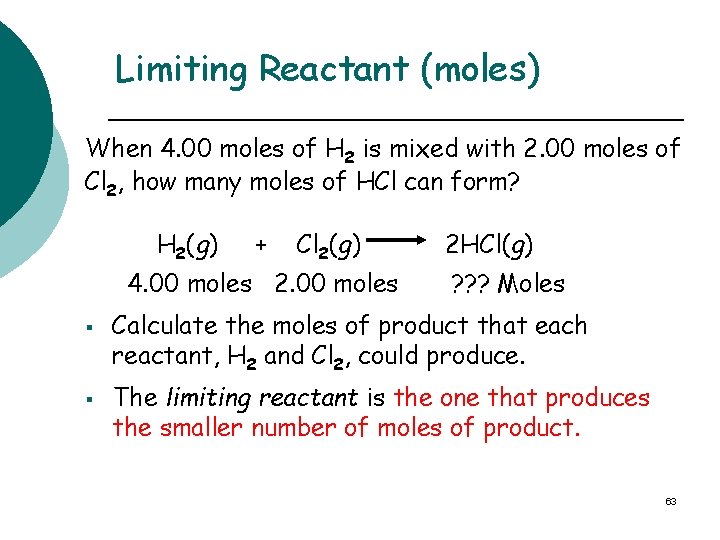
Limiting Reactant (moles) When 4. 00 moles of H 2 is mixed with 2. 00 moles of Cl 2, how many moles of HCl can form? H 2(g) + Cl 2(g) 4. 00 moles 2. 00 moles 2 HCl(g) ? ? ? Moles Calculate the moles of product that each reactant, H 2 and Cl 2, could produce. The limiting reactant is the one that produces the smaller number of moles of product. 63

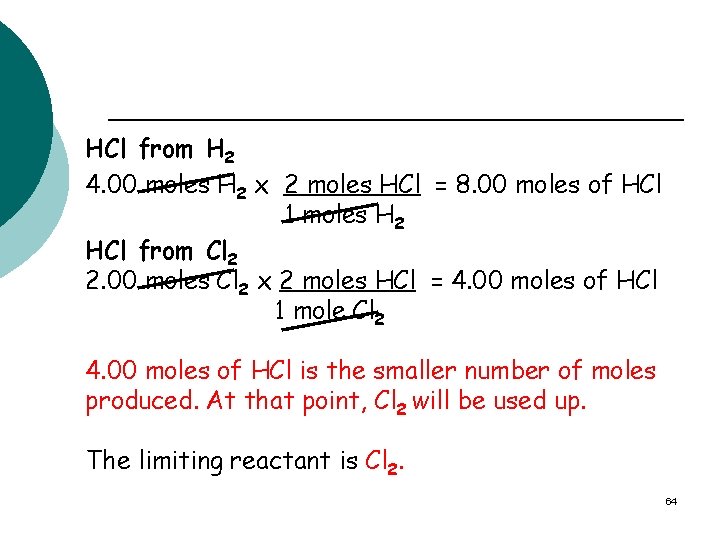
HCl from H 2 4. 00 moles H 2 x 2 moles HCl = 8. 00 moles of HCl 1 moles H 2 HCl from Cl 2 2. 00 moles Cl 2 x 2 moles HCl = 4. 00 moles of HCl 1 mole Cl 2 4. 00 moles of HCl is the smaller number of moles produced. At that point, Cl 2 will be used up. The limiting reactant is Cl 2. 64

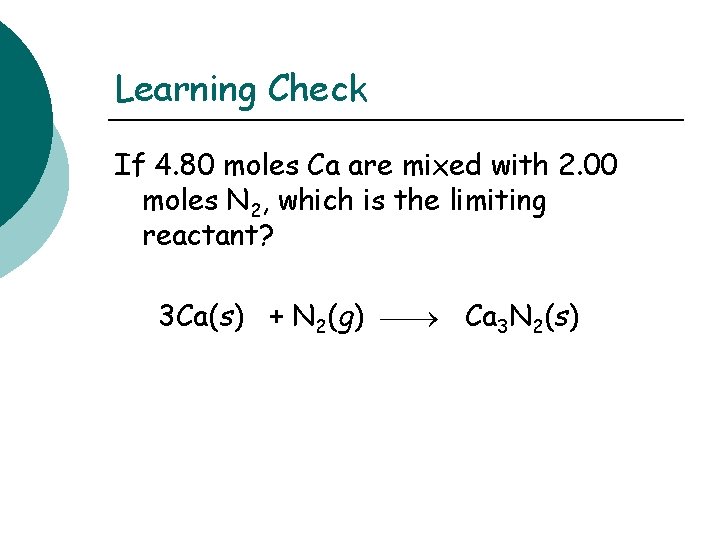
Learning Check If 4. 80 moles Ca are mixed with 2. 00 moles N 2, which is the limiting reactant? 3 Ca(s) + N 2(g) Ca 3 N 2(s)

Solution moles of Ca 3 N 2 from Ca 4. 80 moles Ca x 1 mole Ca 3 N 2 = 1. 60 moles Ca 3 N 2 3 moles Ca moles of Ca 3 N 2 from N 2 2. 00 moles N 2 x 1 mole Ca 3 N 2 = 2. 00 moles Ca 3 N 2 1 mole N 2 Ca is used up when 1. 60 mole Ca 3 N 2 forms. Thus, the limiting reactant is Ca.

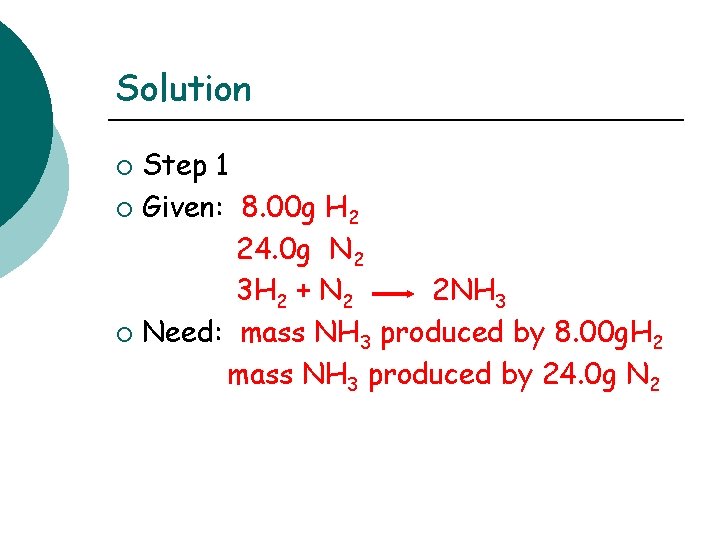
Limiting Reactants Using Mass Problem: What is the mass of ammonia (NH 3) that can be produced when 8. 00 g of H 2 and 24. 0 g of N 2 react? 3 H 2 (g) + N 2 (g) 2 NH 3 (g)

Solution Step 1 ¡ Given: 8. 00 g H 2 24. 0 g N 2 3 H 2 + N 2 2 NH 3 ¡ Need: mass NH 3 produced by 8. 00 g. H 2 mass NH 3 produced by 24. 0 g N 2 ¡

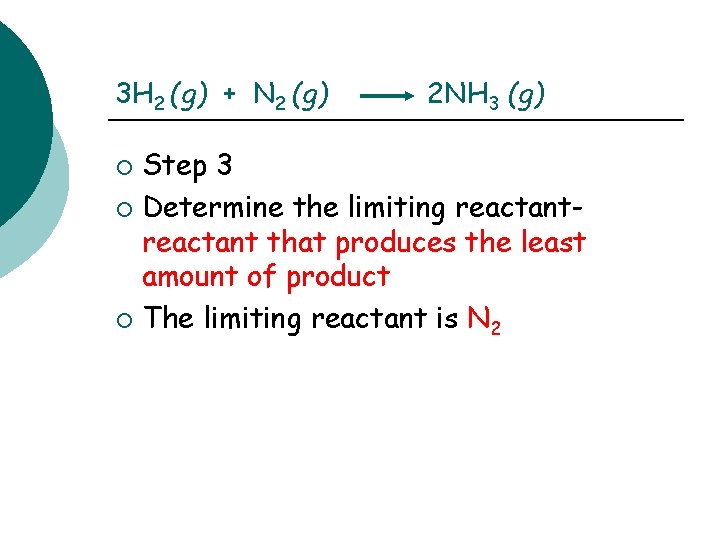
3 H 2 (g) + N 2 (g) 2 NH 3 (g) Step 2: set up & solve step 1 ¡ H 2 ¡ ¡ 8 g. H 2 X 1 mole H 2 X 2 moles NH 3 = 2. 6 moles NH 3 2 g H 2 3 moles H 2 N 2 24 g N 2 X 1 mole N 2 X 2 moles NH 3 = 1. 7 moles NH 3 28 g N 2 1 mole N 2

3 H 2 (g) + N 2 (g) 2 NH 3 (g) Step 3 ¡ Determine the limiting reactant that produces the least amount of product ¡ The limiting reactant is N 2 ¡

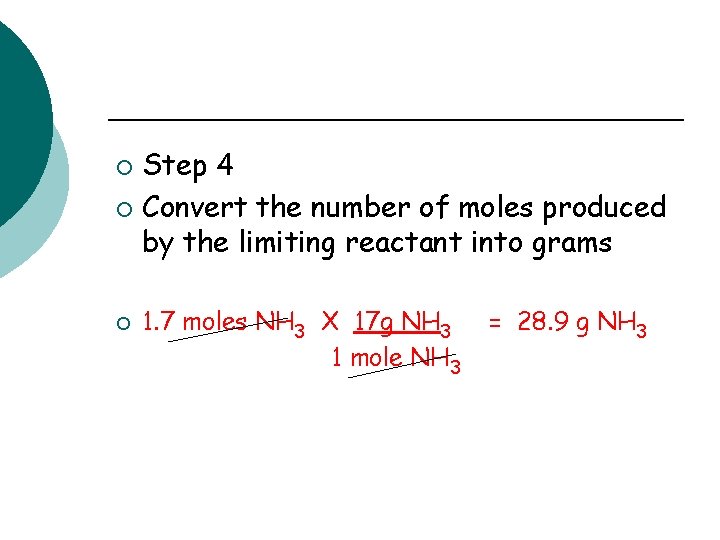
Step 4 ¡ Convert the number of moles produced by the limiting reactant into grams ¡ ¡ 1. 7 moles NH 3 X 17 g NH 3 1 mole NH 3 = 28. 9 g NH 3

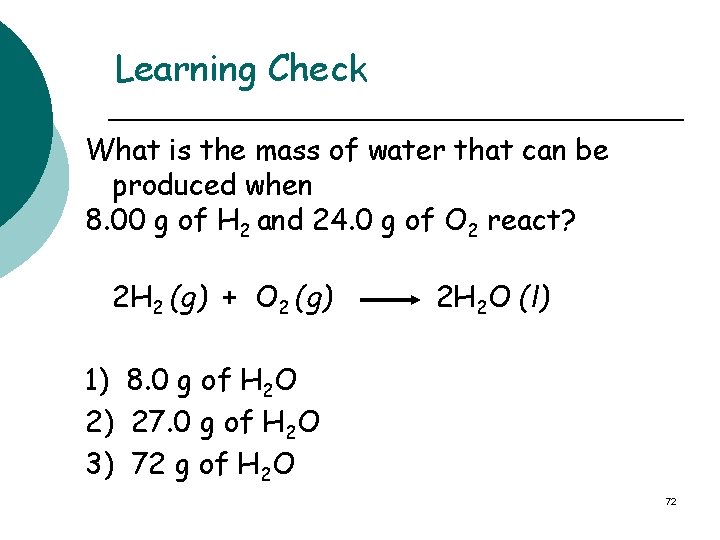
Learning Check What is the mass of water that can be produced when 8. 00 g of H 2 and 24. 0 g of O 2 react? 2 H 2 (g) + O 2 (g) 2 H 2 O (l) 1) 8. 0 g of H 2 O 2) 27. 0 g of H 2 O 3) 72 g of H 2 O 72

Solution Step 1 & 2 Moles of H 2 O from H 2: 8. 00 g H 2 x 1 mole H 2 x 2 moles H 2 O = 4. 0 moles of H 2 O 2. 0 g H 2 2 moles H 2 Moles of H 2 O from O 2: 24. 0 g O 2 x 1 mole O 2 x 2 moles H 2 O = 1. 50 moles of H 2 O 32. 0 g O 2 1 mole O 2

Step 3 ¡ Limiting reactant is O 2 Step 4 – mass of product produced by limiting reactant 1. 50 moles H 2 O x 18. 0 g H 2 O = 27. 0 g of H 2 O 1 mole H 2 O
 Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Chapter 9 modern chemistry review answers
Chapter 9 modern chemistry review answers Chapter 11 stoichiometry answer key
Chapter 11 stoichiometry answer key A balanced chemical equation allows one to determine the
A balanced chemical equation allows one to determine the Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block nhĩ thất độ 1
Block nhĩ thất độ 1 Thể thơ truyền thống
Thể thơ truyền thống Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Tìm vết của mặt phẳng
Tìm vết của mặt phẳng Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Tôn thất thuyết là ai
Tôn thất thuyết là ai Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thermite reaction formula
Thermite reaction formula Stoichiometry is
Stoichiometry is General chemistry 1 stoichiometry
General chemistry 1 stoichiometry Ap chemistry stoichiometry
Ap chemistry stoichiometry Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Chapter 11 stoichiometry
Chapter 11 stoichiometry Chapter 9 review introduction to stoichiometry
Chapter 9 review introduction to stoichiometry What is the first step in most stoichiometry problems?
What is the first step in most stoichiometry problems? Percentage yield
Percentage yield Chapter 12 stoichiometry answer key pearson
Chapter 12 stoichiometry answer key pearson Ideal stoichiometric calculations
Ideal stoichiometric calculations Chapter 3 stoichiometry answer key
Chapter 3 stoichiometry answer key Chapter 12 stoichiometry
Chapter 12 stoichiometry Chapter 11 stoichiometry answer key
Chapter 11 stoichiometry answer key Limiting reactant def
Limiting reactant def Reabsorption
Reabsorption In thermogram the horizontal portion in the curve indicates
In thermogram the horizontal portion in the curve indicates Visceral peritoneum
Visceral peritoneum Standard portion cost formula
Standard portion cost formula Ductnn
Ductnn Portion distortion quiz
Portion distortion quiz Axial wall depth in class 2 amalgam
Axial wall depth in class 2 amalgam Audience of memorandum
Audience of memorandum Longue portion du biceps
Longue portion du biceps Ms word introduction
Ms word introduction Portion of pasta calories
Portion of pasta calories Indented portion of rifling
Indented portion of rifling Face mapping drawing
Face mapping drawing Fourth portion of the duodenum
Fourth portion of the duodenum What is sectional view
What is sectional view Current portion of long-term debt
Current portion of long-term debt Current portion of long term debt
Current portion of long term debt Precision nutrition portion size
Precision nutrition portion size Who of the following was not part of the bebop generation?
Who of the following was not part of the bebop generation? How to calculate edible portion
How to calculate edible portion Parts of small intestine
Parts of small intestine Methods of portion control in bread and pastry
Methods of portion control in bread and pastry Yellowish liquid portion of the blood
Yellowish liquid portion of the blood Inspirational leader mohandas gandhi phrase or clause
Inspirational leader mohandas gandhi phrase or clause Food arrangement on plate
Food arrangement on plate Enigma
Enigma Though my everlasting portion
Though my everlasting portion A movable plate that covers the shuttle
A movable plate that covers the shuttle Calculating food cost
Calculating food cost Lesson 6-2 fractions, decimals, and percents answers
Lesson 6-2 fractions, decimals, and percents answers Calories in 1 cup pasta
Calories in 1 cup pasta Find the area of shaded portion of the given figure.
Find the area of shaded portion of the given figure. Parts of a reflex arc
Parts of a reflex arc What is a eclipse
What is a eclipse What is an eclipse
What is an eclipse Portion controlled cuts
Portion controlled cuts Narrowest part of esophagus
Narrowest part of esophagus Trusses in engineering mechanics
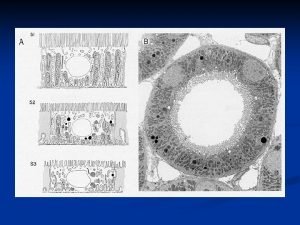
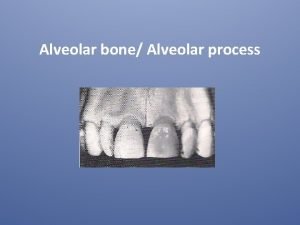
Trusses in engineering mechanics Alveolar portion
Alveolar portion This graph shows a portion of an odd function
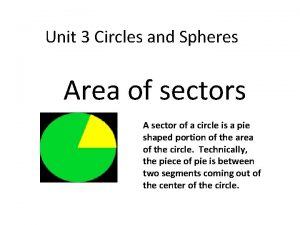
This graph shows a portion of an odd function A pie shaped portion of the circle
A pie shaped portion of the circle The dark inner portion of the shadow cone
The dark inner portion of the shadow cone 1 oz of meat looks like
1 oz of meat looks like The sensing portion of a bi-metallic stem thermometer is:
The sensing portion of a bi-metallic stem thermometer is: The visible, pendant portion of a penis
The visible, pendant portion of a penis