AP Chemistry Chapter 3 Stoichiometry Stoichiometry Is the





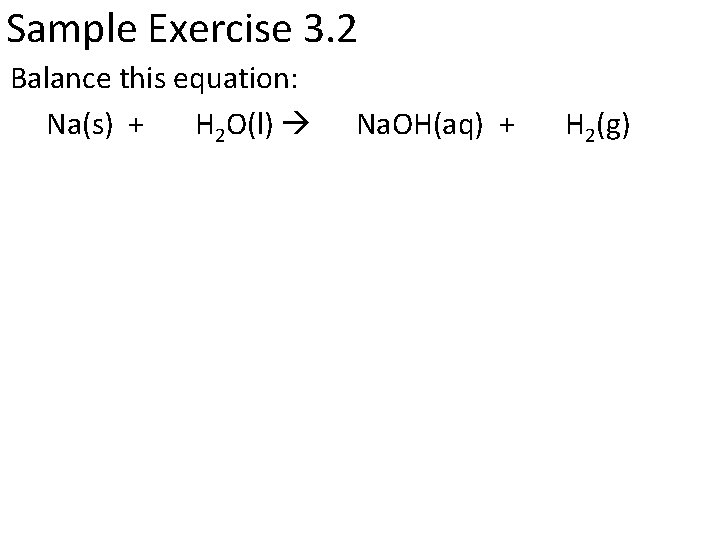





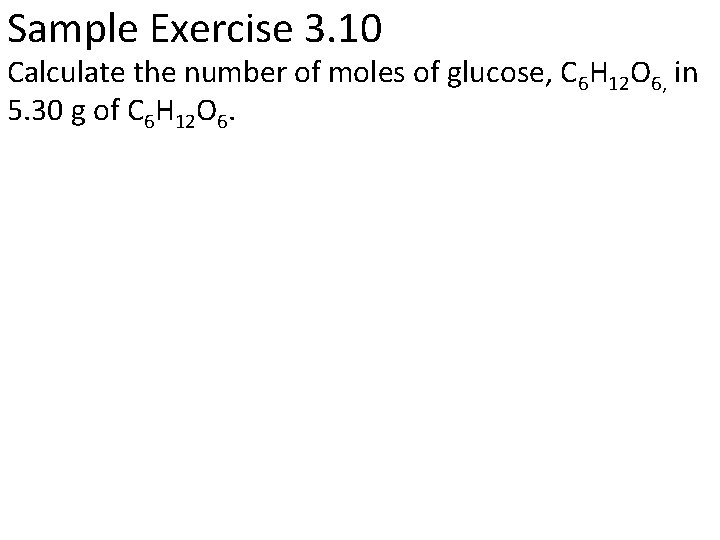

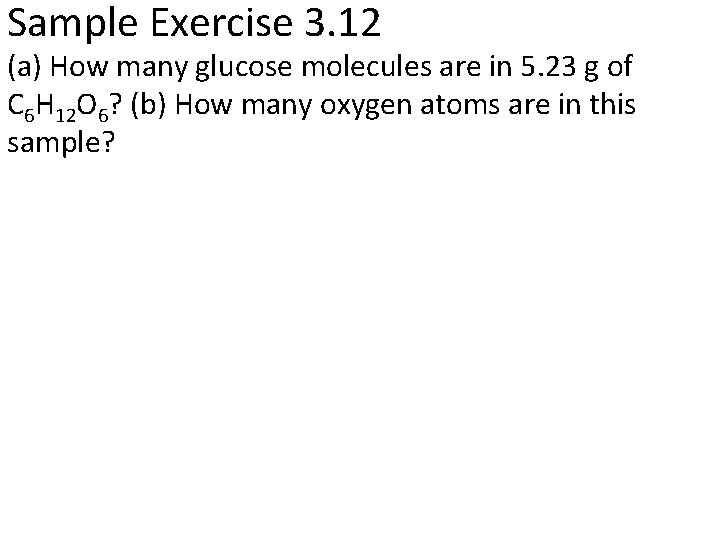









- Slides: 42

AP Chemistry Chapter 3 Stoichiometry

Stoichiometry • Is the study of the quantities of substances consumed and produced in chemical reactions • Derived from the Greek words stoicheion meaning “element” and metron meaning “measure”

Law of Conservation of Mass • Discovered by Antoine Lavoisier in the late 1700’s – Found that the masses of substances after a chemical reaction are equal to the masses of substances before the reaction • Atoms are neither created nor destroyed in a chemical reaction – Reactions rearrange atoms in order to form new substances

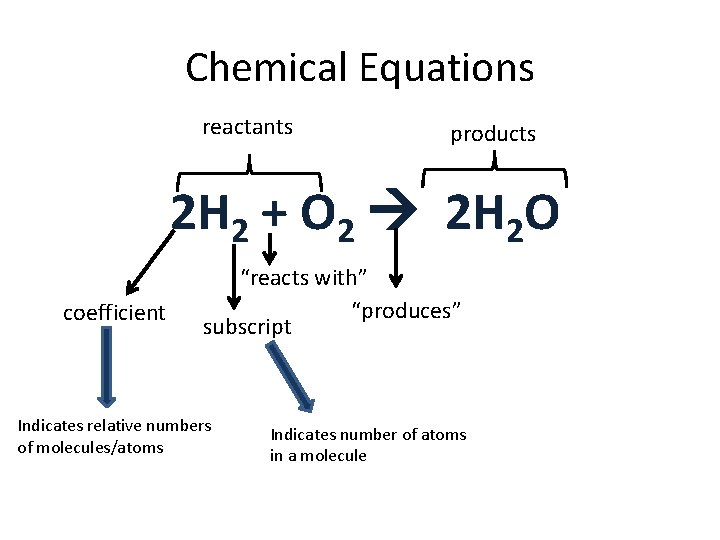
Chemical Equations reactants products 2 H 2 + O 2 2 H 2 O coefficient “reacts with” “produces” subscript Indicates relative numbers of molecules/atoms Indicates number of atoms in a molecule

Balancing Equations • To satisfy the law of conservation of mass • Done by adding or changing coefficients • NEVER balance an equation by changing subscripts!

Balancing Equations Helpful Hints: 1. Start balancing with the elements that appear in the fewest chemical formulas on each side of the equation 2. Balance oxygen last 3. Balance hydrogen next to last

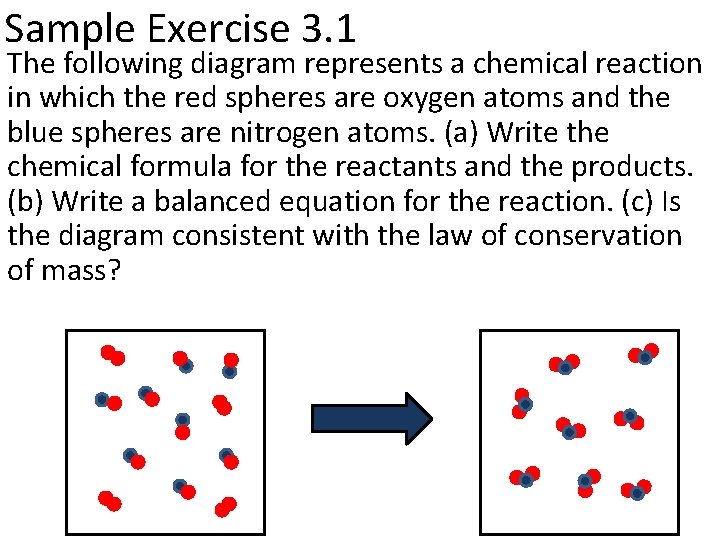
Sample Exercise 3. 1 The following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. (a) Write the chemical formula for the reactants and the products. (b) Write a balanced equation for the reaction. (c) Is the diagram consistent with the law of conservation of mass?

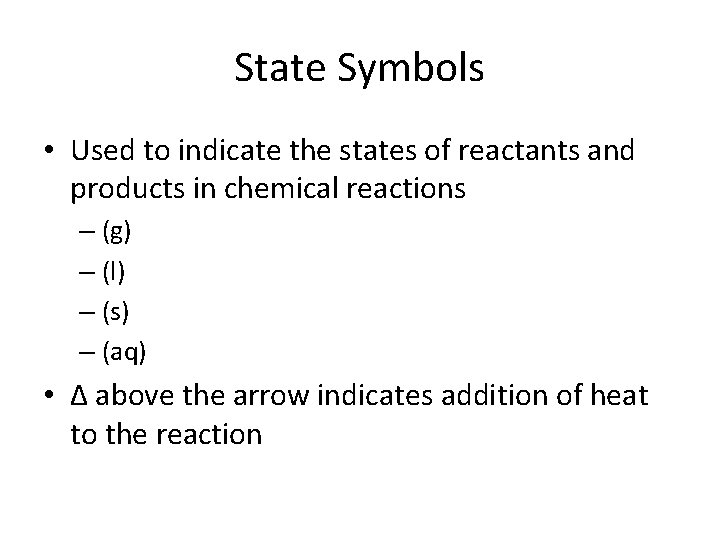
State Symbols • Used to indicate the states of reactants and products in chemical reactions – (g) – (l) – (s) – (aq) • ∆ above the arrow indicates addition of heat to the reaction
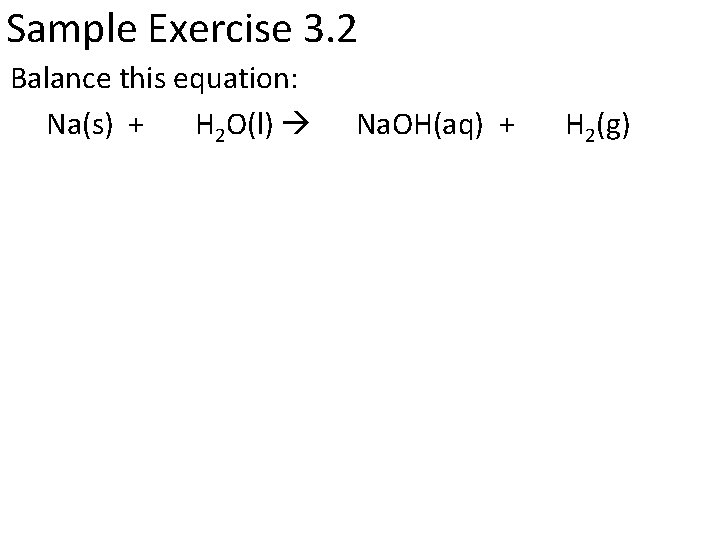
Sample Exercise 3. 2 Balance this equation: Na(s) + H 2 O(l) Na. OH(aq) + H 2(g)

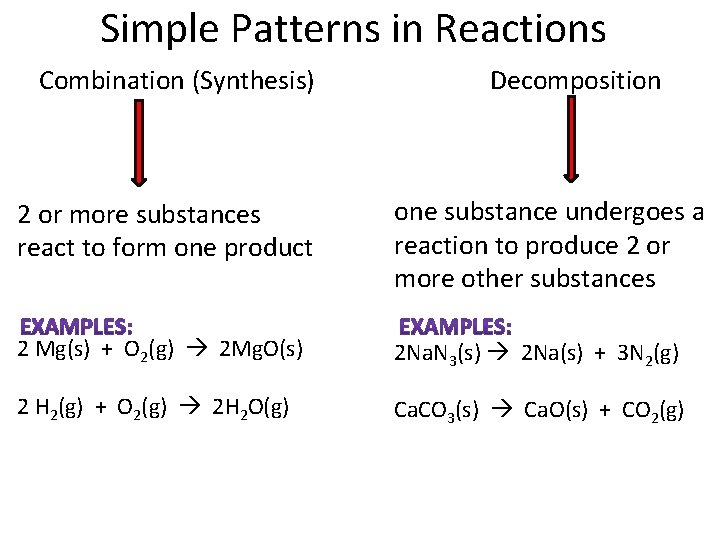
Simple Patterns in Reactions Combination (Synthesis) Decomposition 2 or more substances react to form one product one substance undergoes a reaction to produce 2 or more other substances 2 Mg(s) + O 2(g) 2 Mg. O(s) 2 Na. N 3(s) 2 Na(s) + 3 N 2(g) 2 H 2(g) + O 2(g) 2 H 2 O(g) Ca. CO 3(s) Ca. O(s) + CO 2(g)

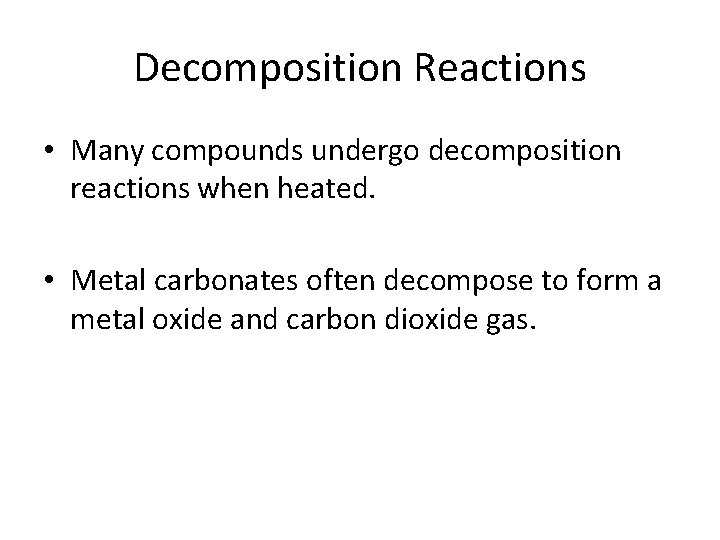
Decomposition Reactions • Many compounds undergo decomposition reactions when heated. • Metal carbonates often decompose to form a metal oxide and carbon dioxide gas.

Sample Problem 3. 3 Write balanced equations for the following reactions: (a) The combination reaction that occurs when lithium metal and fluorine gas react. (b) The decomposition reaction that occurs when solid barium carbonate is heated. (Two products form: a solid and a gas. )

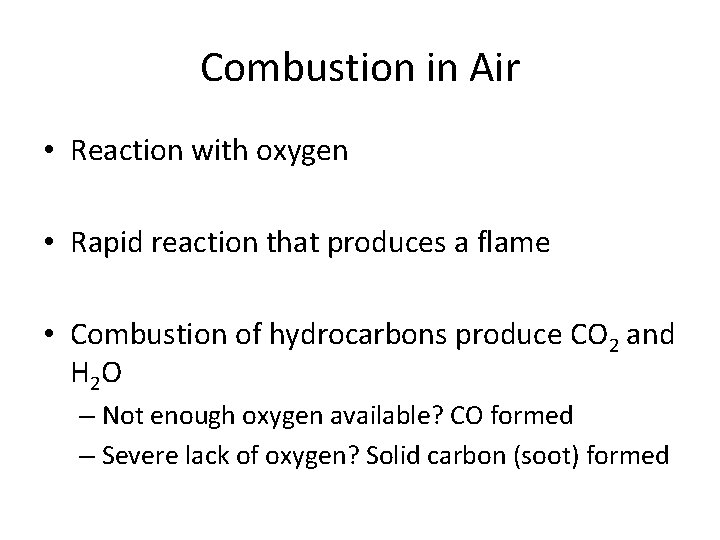
Combustion in Air • Reaction with oxygen • Rapid reaction that produces a flame • Combustion of hydrocarbons produce CO 2 and H 2 O – Not enough oxygen available? CO formed – Severe lack of oxygen? Solid carbon (soot) formed

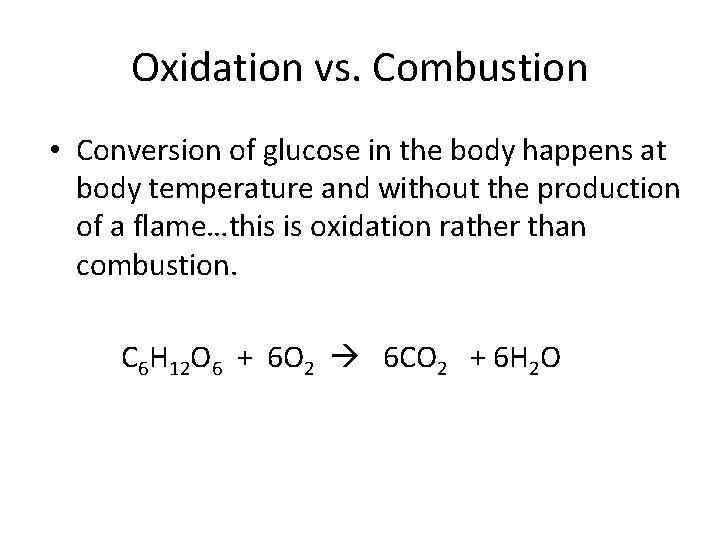
Oxidation vs. Combustion • Conversion of glucose in the body happens at body temperature and without the production of a flame…this is oxidation rather than combustion. C 6 H 12 O 6 + 6 O 2 6 CO 2 + 6 H 2 O

Sample Exercise 3. 4 Write the balanced equation for the reaction that occurs when methanol, CH 3 OH(l), is burned in air.

Formula Weights • Sum of atomic weights of each atom in the chemical formula of a compound • Example: H 2 SO 4 has a formula weight of 98. 1 amu • Atomic weight (for atoms) and molecular weight (for molecular compounds)

Sample Exercise 3. 5 Calculate the formula weight of (a) sucrose, C 12 H 22 O 11, and (b) calcium nitrate, Ca(NO 3)2

Percentage Composition • Percent by mass contributed by each element in the substance

Sample Exercise 3. 6 Calculate the percentage of carbon, hydrogen, and oxygen in C 12 H 22 O 11.

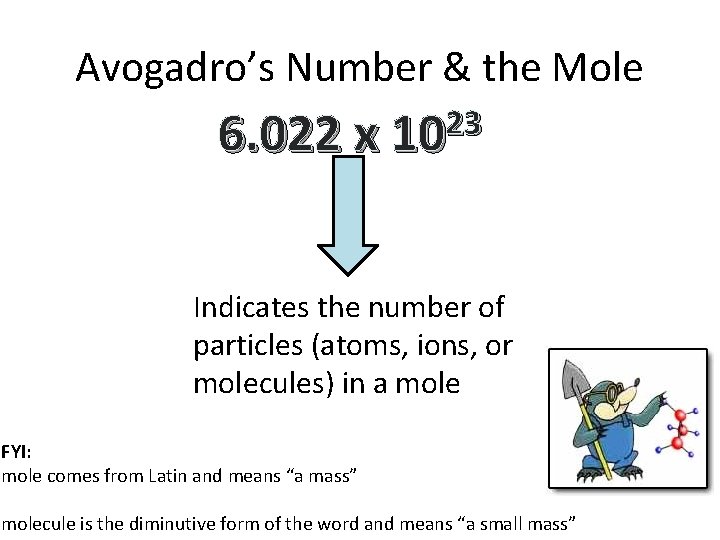
Avogadro’s Number & the Mole 23 6. 022 x 10 Indicates the number of particles (atoms, ions, or molecules) in a mole FYI: mole comes from Latin and means “a mass” molecule is the diminutive form of the word and means “a small mass”
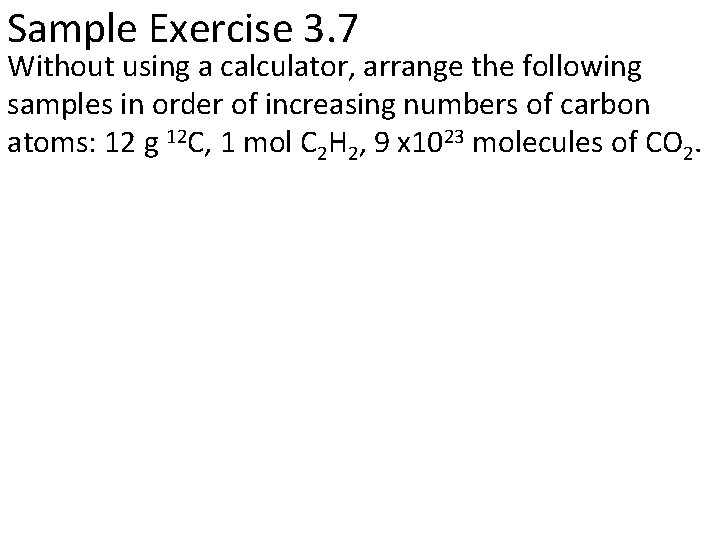
Sample Exercise 3. 7 Without using a calculator, arrange the following samples in order of increasing numbers of carbon atoms: 12 g 12 C, 1 mol C 2 H 2, 9 x 1023 molecules of CO 2.

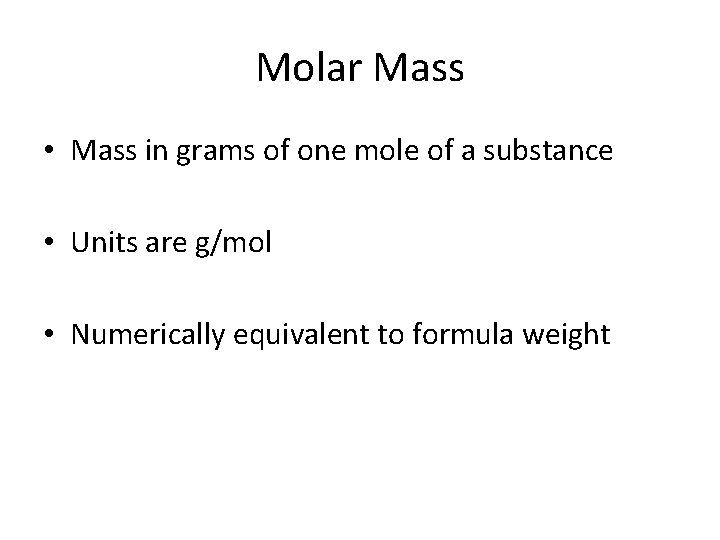
Molar Mass • Mass in grams of one mole of a substance • Units are g/mol • Numerically equivalent to formula weight

Sample Exercise 3. 9 What is the mass in grams of 1. 00 moles of glucose, C 6 H 12 O 6?
Sample Exercise 3. 10 Calculate the number of moles of glucose, C 6 H 12 O 6, in 5. 30 g of C 6 H 12 O 6.

Sample Exercise 3. 11 Calculate the mass, in grams, of 0. 433 mol of calcium nitrate.

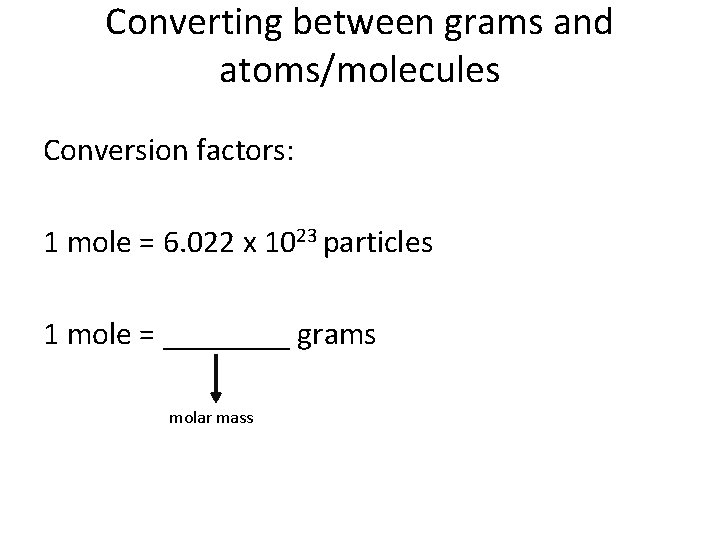
Converting between grams and atoms/molecules Conversion factors: 1 mole = 6. 022 x 1023 particles 1 mole = ____ grams molar mass
Sample Exercise 3. 12 (a) How many glucose molecules are in 5. 23 g of C 6 H 12 O 6? (b) How many oxygen atoms are in this sample?

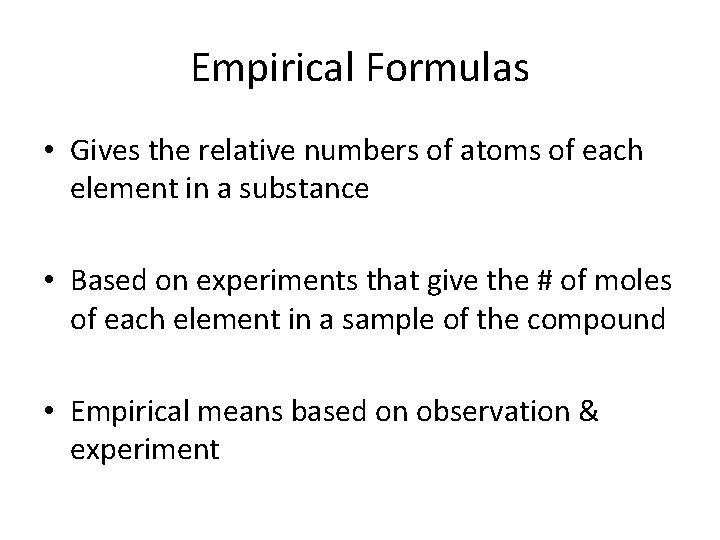
Empirical Formulas • Gives the relative numbers of atoms of each element in a substance • Based on experiments that give the # of moles of each element in a sample of the compound • Empirical means based on observation & experiment

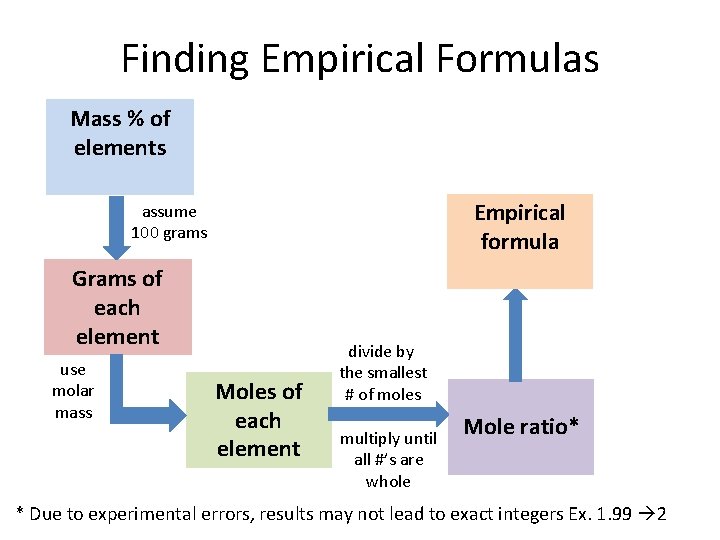
Finding Empirical Formulas Mass % of elements Empirical formula assume 100 grams Grams of each element use molar mass Moles of each element divide by the smallest # of moles multiply until all #’s are whole Mole ratio* * Due to experimental errors, results may not lead to exact integers Ex. 1. 99 2

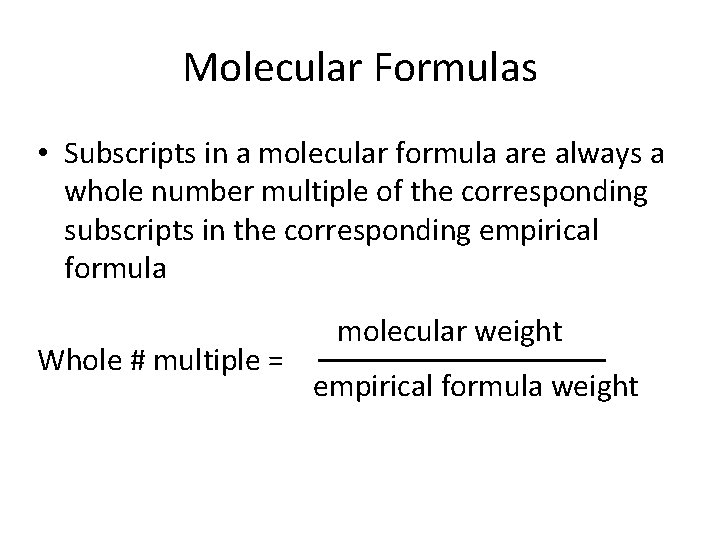
Molecular Formulas • Subscripts in a molecular formula are always a whole number multiple of the corresponding subscripts in the corresponding empirical formula Whole # multiple = molecular weight empirical formula weight

Sample Exercise 3. 13 Ascorbic acid (vitamin C) contains 40. 92% C, 4. 58% H, and 54. 50% O by mass. What is the empirical formula of ascorbic acid?

Sample Exercise 3. 14 Mesitylene, a hydrocarbon that occurs in small amounts in crude oil, has an empirical formula of C 3 H 4. The experimentally determined molecular weight of the substance is 121 amu. What is the molecular formula of mesitylene?

Sample Exercise 3. 15 Isopropyl alcohol, a substance sold as rubbing alcohol, is composed of C, H, and O. Combustion of 0. 255 g of isopropyl alcohol produces 0. 561 g of CO 2 and 0. 306 g of H 2 O. Determine the empirical formula of isopropyl alcohol.

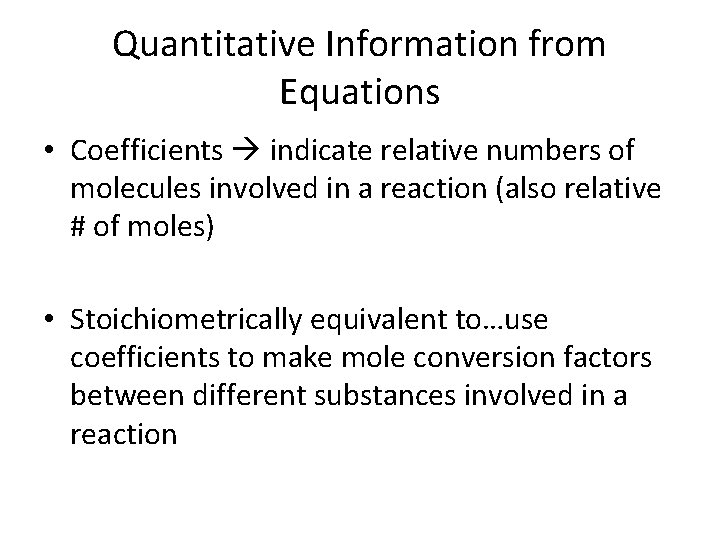
Quantitative Information from Equations • Coefficients indicate relative numbers of molecules involved in a reaction (also relative # of moles) • Stoichiometrically equivalent to…use coefficients to make mole conversion factors between different substances involved in a reaction

Sample Exercise 3. 16 How many grams of water are produced in the oxidation of 1. 00 g of glucose, C 6 H 12 O 6?

Sample Exercise 3. 17 Solid lithium hydroxide is used in space vehicles to remove exhaled carbon dioxide. The lithium hydroxide reacts with gaseous carbon dioxide to form solid lithium carbonate and liquid water. How many grams of carbon dioxide can be absorbed by 1. 00 gram of lithium hydroxide?

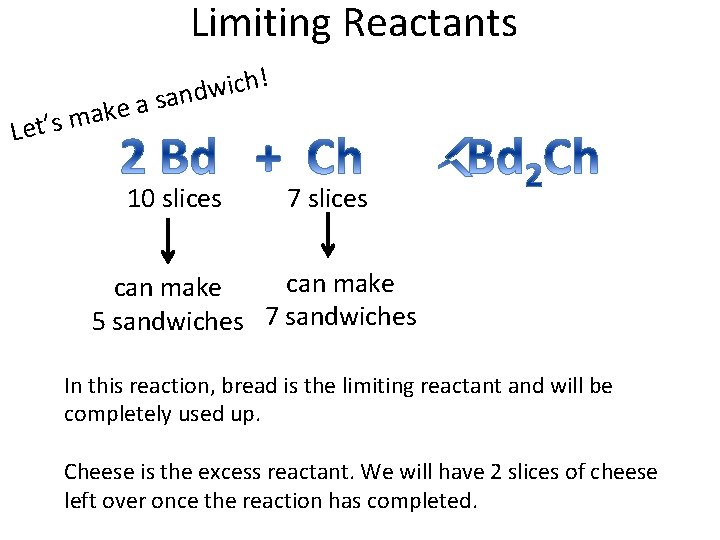
Limiting Reactants ! h c i w d n a s a e ak m s ’ t Le 10 slices 7 slices can make 5 sandwiches 7 sandwiches In this reaction, bread is the limiting reactant and will be completely used up. Cheese is the excess reactant. We will have 2 slices of cheese left over once the reaction has completed.

Limiting Reactants • Also called limiting reagents • In a chemical reaction, the reaction stops when one reactant is totally used up. This is the limiting reactant.

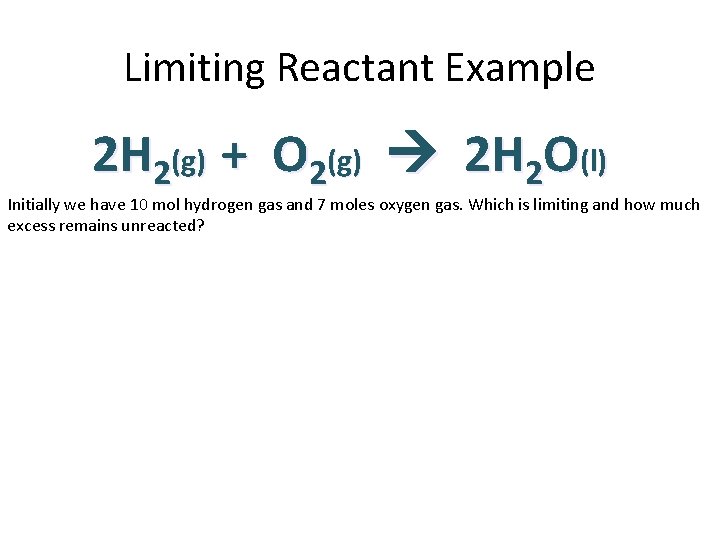
Limiting Reactant Example 2 H 2(g) + O 2(g) 2 H 2 O(l) Initially we have 10 mol hydrogen gas and 7 moles oxygen gas. Which is limiting and how much excess remains unreacted?

Sample Exercise 3. 18 The most important commercial process for converting N 2 from the air into nitrogen-containing compounds is based on the reaction of N 2 and H 2 to form ammonia (NH 3): N 2(g) + 3 H 2(g) 2 NH 3(g) How many moles of NH 3 can be formed from 3. 0 mol of N 2 and 6. 0 mol of H 2?

Sample Exercise 3. 19 Consider the following reaction: 2 Na 3 PO 4 (aq) + 3 Ba(NO 3)2(aq) Ba 3(PO 4)2 (s) + Na. NO 3 (aq) Suppose a solution containing 3. 50 g of Na 3 PO 4 is mixed with a solution containing 6. 40 g of Ba(NO 3)2. How many grams of Ba 3(PO 4)2 can be formed?

Sample Exercise 3. 20 Adipic acid, H 2 C 6 H 8 O 4 , is used to produce nylon. The acid is made commercially by a controlled reaction between cyclohexane (C 6 H 12) and O 2: 2 C 6 H 12 (l) + 5 O 2(g) 2 H 2 C 6 H 8 O 4 (l) + 2 H 2 O (g) (a) Assume that you carry out this reaction starting with 25. 0 g of cyclohexane and that cyclohexane is the limiting reactant. What is theoretical yield of adipic acid? (b) If you obtain 33. 5 g of adipic acid from your reaction, what is the percent yield of adipic acid?